Wow, real life really kicked my ass there. I'll try and post a couple of things a month again from now on, although posts may be shorter for a while! Thanks for still reading! —BRSM
An Enantiospecific Synthesis of Jiadifenolide
Erik J. Sorensen et al., 2014, 53, 5332–5335 [PDF] [SI] [Group]
Given the massive number of people affected worldwide by neurodegenerative diseases and nerve injuries, it's not surprising that a number of synthetic groups have chosen to focus their research programs on neurotrophic[1] natural products. Of course, it's probably coincidence that aside from their potential uses to society, a number of these compounds seem to also be structurally unique and strikingly intricate molecules.[2] One such example is jiadifenolide, whose dense, caged seco-prezizaane-type structure has already seen 3 total syntheses since its isolation five years ago.
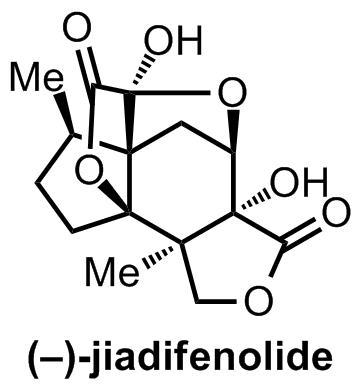
Now, there’s a saying in the field that a synthesis should strive to either be the first, or be the best (or sometimes "last", because no-one else will be able to do a better job). At any rate, it's certainly true that when a target's been made more than a couple of times, those are certainly the ones that people are more likely to remember. In this case, the impressive first synthesis of the target was achieved by Theodorakis and coworkers at UC San Diego back in 2011, but at 25 steps and 1.5% overall yield, it appeared that some in the community felt that the title of "last" was still very much in contention. Indeed, with syntheses from the Sorensen and Dalby/Paterson groups in the last few months, it seems that interest in the jiadifenolide problem is still strong.
I'd initially planned to write about the most recent 2 (or possibly all 3) syntheses in an epic all-in-one comparison blog-post, but in the interests of keeping these musings short and somewhat readable I've decided to break things down a bit. This week's installment will cover Sorenson's awesome synthesis from back in April.
The Sorenson group obtained their desired enantiopure building block from the chiral pool, via three known, decagram scale manipulations on (R)-pulegone— which itself definitely falls into the ‘easily affordable’ bracket of commercial starting materials. [3]
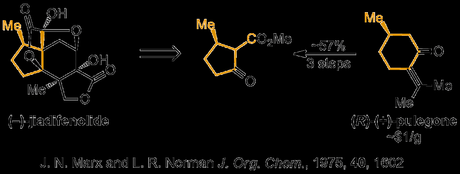
Taking their chiral β-ketoester building block, the group proceeded to use a substrate-controlled Robinson annulation to append jiadifenolide’s central cyclohexane ring with near-perfect diastereoselectivity. This was followed up with another old-school reaction—a deconjugative double alkylation of the enone—using conditions cribbed from R. B. Woodward’s 1954 lanesterol synthesis.[4] The ketone was then protected, and the ester converted into the aldehyde. Next, the group needed to extend this aldehyde out by one carbon atom to form the molecule’s bridging lactone, but unfortunately the usual gamut of homologation reactions was unsuccessful, presumably due to the steric crowding around the neopentyl reaction site. Eventually, they found that the rather uncommon van Leusen reaction could be used to homologate the aldehyde to a nitrile group in excellent yield, and heating the product in sulfuric acid and methanol effected both hydrolysis and lactonisation onto the nearby double bond.
At this point came the riskiest part of the group’s strategy; diastereoselective oxidation of the one of the two geminal dimethyl groups on the cyclohexyl ring to allow construction of the second lactone. In their retrosynthesis the group had envisioned the use of some Sanford-type palladium(IV) acetoxylation chemistry, thus needed to install a suitable directing group—which they did in the form of an oxime.

Unfortunately, no diastereoselectivity for the desired β-methyl group was observed and a 1:1 mixture of epimeric acetates was obtained in all cases—along with small amounts of the doubly-oxidised product. However, it's important to note that despite a somewhat low yield of the required diastereomer, the scalability of the reaction—and the previous steps—meant that grams of the desired product could still be obtained and the group could push on. Thus, having served its purpose as a directing (or at least, enabling) group, the oxime was now reductively cleaved back to the ketone, which converted to the corresponding vinyl triflate using Comins’ reagent. Subsequent palladium-mediated methoxycarbonylation, followed by acetate deprotection—leading to spontaneous cyclisation—completed the target’s second lactone in short order. Unfortunately, a mixture of three compounds was obtained from this sequence: the desired lactone, along with isomerised starting material and a methanol adduct (resulting from oxa-Michael addition to the α,β-unsaturated ester). However, the group conveniently found when this mixture was subjected to classic Weitz–Scheffer epoxidation conditions with basic peroxide, a single oxirane was obtained in good yield. With the end now in sight, oxidation of the enolisable lactone was required for closure of the final ring and to install the requisite keto group the team chose a rather unusual Pummerer-like rearrangement recently reported by Danishefsky.[5] This interesting tactic involved an initial α-iodination of the lactone carbonyl (via the silyl ketene acetal), followed by treatment of the resulting iodide with DMDO to give the putative iodoso compound, which then rearranged to the desired α-ketoester. Finally, treatment of this compound with lithium hydroxide—followed by an acidic work up—installed the bridging oxygen atom and completed the natural product.
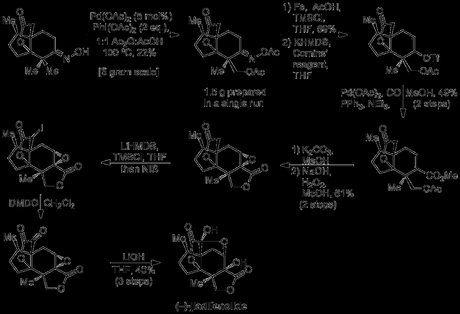
Looking back on this post, it’s pretty striking how different the two schemes above are in terms of the chemistry they contain. There’s nothing in the first half of the synthesis that would look out of place in a classic 1950’s steroid synthesis (apart from maybe the DIBAL), but the methyl group oxidation is a very interesting (and bold) disconnection, and the iodoso-Pummerer rounds things off nicely. Well done guys!
Addenda
1. The -trophic suffix is from the Greek trophē, "nourishment". I really gotta get round to another etymology blog post.
2. This field was reviewed by Theodorakis earlier in the year. In particular, huperzine a (I wrote about Shair's synthesis a couple of years back), and merrilactone have inspired some creative chemistry.
3. In contrast to the Theodorakis group, who ultimately disconnected the molecule back to a very Hajos–Parrish-like starting material, which they synthesised with some efficient proline-based organocatalysis.
4. In fact the group draw conditions from several classic golden-era steroid papers
5. See Org. Lett. 2000, 2, 3493
