Stereoselective Total Synthesis of Hainanolidol and Harringtonolide via Oxidopyrylium-Based [5 + 2] Cycloaddition
Weiping Tang et al., J. Am. Chem. Soc., 2013, ASAP; [PDF] [SI] [GROUP]
DOI: 10.1021/ja406255j
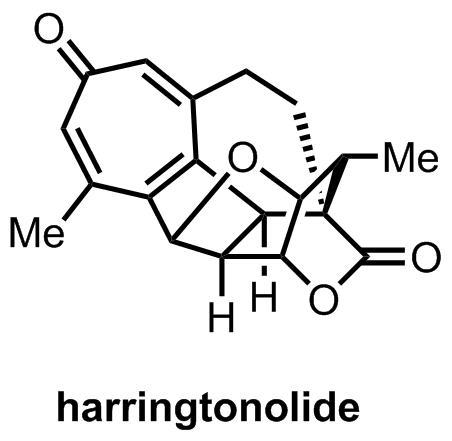
Everyone who's studies organic chemistry long enough has a favorite reaction or two, although unusually in my case I’ve never actually performed either of mine. One is the alkene–arene metaphotocycloaddition that I wrote about last year for Carmen’s IYC2011 Favourite Reaction Carnival, first discovered by Bryce-Smith (in Reading, UK, of all places) and sharpened into a useful synthetic tool by Wender, Mulzer and others. The second is probably the [5 + 2] oxidopyrylium cycloaddition, a handy way of making 7-membered rings with nary a metal in sight.[1] Neither is particularly common in total synthesis, so imagine my delight when I saw the latter featured in Tang’s recent synthesis of harringtonolide a couple of weeks back.
The target in question comes from the Cephalotaxus genus of plants, which—by means of the incredibly popular cephalotaxine and harringtonine alkaloids—has provided synthetic chemists with a great deal of entertainment over the past 50 years or so. It’s interesting to note that the Cephalotaxus genus itself belongs to the larger family Taxaceae, which also encompasses the yew tree Taxus baccata, well known to natural products chemists as the original source of the famous microtubule stabiliser and anti-cancer drug taxol. Well, it seems that humankind has again struck gold in the Taxaeae family as harringtonolide has recently been demonstrated to be a remarkable potent and selective anti-neoplastic agent. But enough on taxol and taxonomy—let’s talk synthesis!
The group’s plan relied on the use of the aforementioned [5 + 2] oxidopyrylium cycloaddition to construct the seven membered ring. This clever, central disconnection essentially reduces the rather intimidating carbon skeleton of harringtonolide to a comparatively simple problem in decalin synthesis and—although it's a rather strange looking species—the precursor to the oxidopyrylium required to pull it off is just a simple furan.
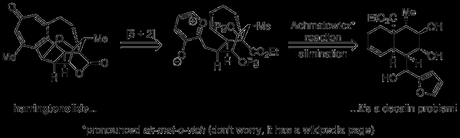
The route began with the bicyclic enone shown below, and it probably won’t surprise anyone to learn that this was synthesised using the famous Robinson annulation, although at first glance it does seem a little odd that the racemic form of this building block was used, given that this reaction is usually easily rendered enantioselective with a little organocatalysis.[2] However, on closer inspection it turns out that—although no comment is made in the paper itself—if one delves into the SI the group does detail their unsuccessful search for a suitable catalyst for this transformation; in total synthesis even 'general' reactions are often far from universal!
Pushing on with the racemic route, the enone was γ-hydroxylated in a vinylogous Rubottom-type oxidation involving diene formation, followed by treatment with oxone. A 4:1 mixture of diastereomers was obtained (favouring the unwanted epimer!), and this mixture was carried though the next four steps. A second Rubottom-type reaction—involving silyl enol ether formation, followed by treatment with osmium tetroxide—was then used to α-hydroxylate the other side of the ketone.[3] Having served its purpose of activating the nearby methylene groups for oxidation, the ketone was now reduced to give the trans-diol, which was then protected as the bis(TBS ether).[4] Now, with the conformational preference of the ring system sufficiently altered by these events, the incorrectly set stereocentre from the first oxidiation could finally be epimerized. Thus, deprotection, oxidation and reduction gave the alcohol allyic alcohol with better than 20:1 diastereoselectivity in favour of the desired compound, and this stereochemical information was now transferred to the righthand ring by means of a of a Claisen rearrangement. This was performed by the now-justifiably-uncommon method of mercury trifluoroacetate-catalysed vinyl transfer, followed by heating the vinyl ether obtained in refluxing toluene. The resulting aldehyde was then converted to the corresponding Weinreb amide in order to allow installation of the furan ring at a later stage, and finally an allylic oxidation was carried out to give the enone shown below.
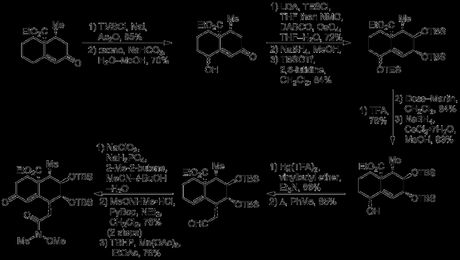
Although the introduction of a carbonyl group into the left-hand ring may seem counterintuitive (as no oxygenation is ultimately required in this half of the bicycle), it was hoped that its presence would facilitate formation of the thermodynamically unfavourable cis-decalin. It was anticipated—correctly—by the group that reduction of this carbonyl—or its hydrazone—would occur via hydride delivery to the top face of the molecule and they exploited this reactivity to generate an diazene intermediate that underwent [3,3]-sigmatropic rearrangement set the remaining ring junction stereocentre with the required stereochemistry.[5] Next, the furan was introduced by addition of 2-furylmagnesium bromide to the Weinreb amide, and it was now time to test the key [5 + 2] cycloaddition. First, oxidative expansion of the furan had to be carried out; this was achieved by reduction of the adjacent ketone to the secondary alcohol, which was then used to direct Achmatowicz oxidation of the furan itself with tert-butylhydroperoxide and a vanadium catalyst. The oxidation and rearrangement produced the expected hydroxypyranone in good yield, and this was then acetylated with acetic anhydride in pyridine. Finally, heating this somewhat unstable intermediate with DBU in refluxing chloroform lead to oxidopyrylium formation (via elimination of acetic acid and tautomerisation) and the anticipated cycloadduct was isolated in excellent yield.[6]
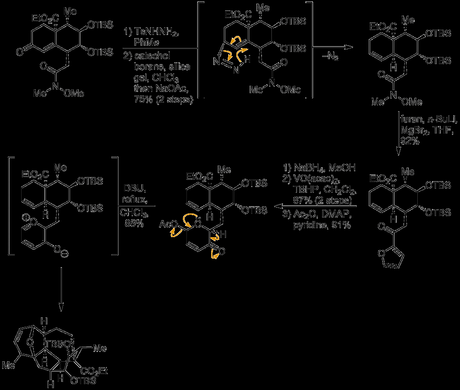
Now that the key step had successfully been realised, the group faced the task of converting the oxabicyclooctane ring fromed by the [5 + 2]-cycloaddition into the tropone ring found in the natural product.[7] First, the additional methyl group required was introduced by simple addition of methyl magnesium bromide to the enone under substrate control. This was then followed by selective removal of one of the TBS groups using trichloroacetic acid, and lactonisation. Now—at least according to the group’s initial plan—only a couple of steps remained; a Dauben reaction was used to oxidatively transpose the newly formed tertiary allylic alcohol to the enone and conditions were now investigated for the removal of the unwanted oxygen bridge. Unfortunately, no direct way of dehydrating the oxabicyclooctane directly to the tropone could be found, and a new endgame had to be devised to sidestep this problem.
Going back a step, the tertiary alcohol that had been oxidised in the previous attempt was instead treated with thiophenol and boron trifluoride etherate, resulting in SN2’ displacement to give the allyl thioether. Removal of the sulfide α-proton using LDA in THF/HMPA then resulted in cleavage of the ether bridge by elimination. Surprisingly, only one of the two possible isometric dienes—the desired one—was formed, and in excellent yield – the route was back on track!
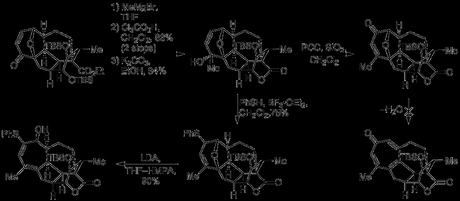
Having served its purpose, the thioether was now removed over a three step sequence involving protection of the vicinal alcohol, oxidation to the sulfoxide and reductive removal by C–S cleavage with samarium diiodide (pepped up with a little DMPU/MeOH). Introduction of the tropone oxygen was then carried out by [4 + 2] cycloaddition of the cycloheptadiene with singlet oxygen, followed by a DBU mediated Kornblum–DeLaMare rearrangement to the enone.[8] Finally, treatment with p-TSA cleaved the TES group and effect a double dehydration to form the much sought after tropone to give the alcohol in good yield. It turns out that this penultimate compound is actually a natural product in its own right and—as its (biomimetic) conversion to harringtonolide had already been demonstrated in the lab—the group had now achieved the formal total synthesis of their target. However—with barely enough material in hand—they still attempted to perform the final reaction themselves, eventually producing 0.2 mg of the target!
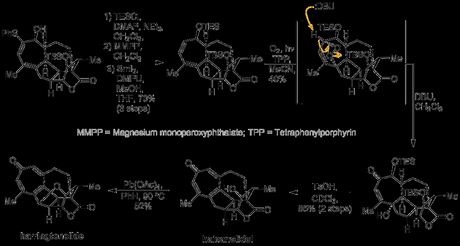
Although its length, linear nature and low overall yield do detract a little, and the final path traveled was somewhat circuitous, I—for one—enjoyed the journey. Well done guys!
Etc.
- There was a dedicated review on this reaction in Tetrahedron a few years back (Tetrahedron, 2008, 64, 3405). A more general review on the [5 + 2] reaction was published by Jeffery Stryker at the start of this year, covering the full gamut of metal catalysed versions as well (Chem. Rev., 2013, 113, 2244).
- In fact, the Hajos–Parrish–Eder–Sauer–Wiechert proline-catalysed aldol reaction used to prepare such substrates is generally regarded as the seminal example of useful asymmetric organocatalysis in the literature. There are many, many reports of this type of cascade in asymmetric decalone synthesis, and of course the reaction is used to make the famous Wieland–Miescher ketone, which has been the starting point for countless (well, several) total syntheses. If I wanted to point out another link to taxol, I could tell you that Danishefsky used the Wieland–Miescher ketone as the starting point for his epic total synthesis.
- The DABCO’s just there to accelerate the dihydroxylation; the reaction’s pretty slow without ligands on the osmium – just ask Barry Sharpless. Quinuclidine also does this job well.
- Curiously, when this reaction was performed with sodium triacetoxyborohydride—a reagent that normally gives trans-diols by intramolecular delivery of hydride—only the cis-diol was obtained (as evidenced by its conversion to the corresponding acetonide. Fortunately, sodium borohydride displayed excellent selectivity for the required trans-diol.
- A variant of this trick has been recently used by Myers and Mulzer.
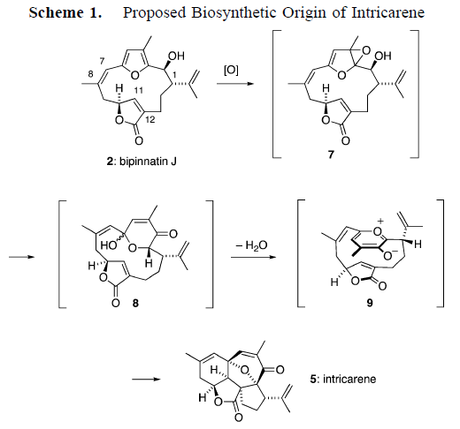
- Obviously in this synthesis this step is not biomimetic, but there are a few cases where this reaction is thought to occur in Nature. The most interesting one that I can think of right now is in the biosynthesis of the natural product intricarene, whose name is cognate with the English word ‘intricate’, thanks to that tortuous structure. The proposed cycloaddition links it to a cembranoid co-isolate, bipinatin J, and has successfully been mimicked in the lab by the groups of Pattenden and Trauner.
7. Although tropone is probably one of the first aromatic carbocyclic compounds that everyone thinks of after benzene (well, apart from maybe Tristan Lambert) it wasn’t until Dewar’s work in 1945 that it occurred to anyone that aromatic rings can come in all shapes and sizes. Dewar was very interested in ‘non-standard’ aromatic compounds, and Dewar Benzene is named for him, as I discussed here.
8. When was the last time you saw one of those so late in a big total synthesis? I'm thinking Nicolaou's 2011 route to epicoccin G (J. Am. Chem. Soc., 2011, 133, 8150), although I dimly recall one in a taxol synthesis... maybe Kuwajima's?
