 The NEJM has come out with a very interesting paper: Evaluation of Convalescent Plasma for Ebola Virus Disease in Guinea. The explosive outbreak of Ebola Virus Disease in West Africa last year had hijacked the headlines and media space in a big way. Multiple solutions were touted, including the vaccine trial STRIVE. Few articles, however, looked at the systems response to the crisis. Although the current study maintains that trend, yet, it plays with a therapeutic ploy which has the potential to become the breakthrough approach in raising generic response to similar disease outbreaks.
The NEJM has come out with a very interesting paper: Evaluation of Convalescent Plasma for Ebola Virus Disease in Guinea. The explosive outbreak of Ebola Virus Disease in West Africa last year had hijacked the headlines and media space in a big way. Multiple solutions were touted, including the vaccine trial STRIVE. Few articles, however, looked at the systems response to the crisis. Although the current study maintains that trend, yet, it plays with a therapeutic ploy which has the potential to become the breakthrough approach in raising generic response to similar disease outbreaks.
Before you go any further in this account, you should check out the Now@NEJM article written by my dear friend Bhavna Seth if you are interested in a more balanced, more authoritative review of the study. Mine is totally colored by my own biases!
This article makes the epidemiologist-in-training in me squirm with discomfort, while the medical romantic in me just breaks out into a jig. It is an elegant study, designed and conducted in the eye of the storm: in the middle of the Ebola Outbreak in Guinea. As someone with a little bit of experience of handling small scale outbreak-situation cases of gastroenteritis and dengue, I know how difficult it is to keep the clinical scaffoldings held up in the middle of such crises – imagining a full scale study in the middle of all this: simply astounding!
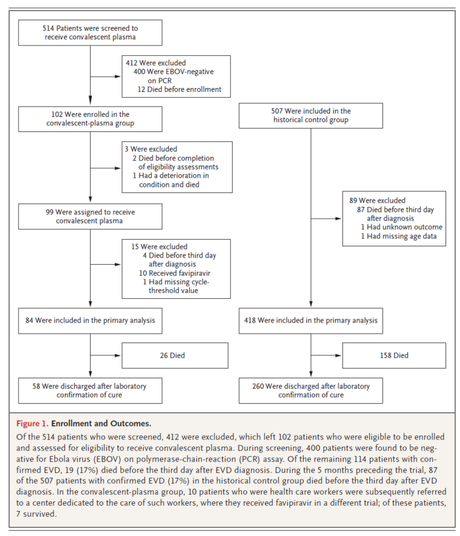
Anyway, the study is methodologically seriously flawed in a couple of ways. Most obvious of these is the fact that the control and intervention groups are, technically speaking, not comparable, but it gives us some insight into the business of managing, or at least stemming the tide of a violent outbreak of infectious diseases. Another major concern is the “goodness” of the plasma being used to treat the patients as none of them were checked for antibody titers. Given that our knowledge of the immunology of Ebola is emerging and taking shape only recently, this is a field that needs further enquiry.
Eventually, however, the study fails to establish the role of convalescent plasma as a therapeutic choice, but, I think a part of that could be attributed to the fact that the study design is slightly flawed, as pointed out earlier. One of the biggest concerns of launching investigations into therapeutic options using blood and blood products is the risk of adverse effects, some of which can be immediately lethal. This study at least showed reassuring evidence that the adverse event rates were much lower than expected when using such treatment plans.
Read it: it makes for a surprisingly effortless read compared to the pharmaceutical-sponsored trials we read nowadays. The simplicity of the design is its strength (and weakness!). So, to conclude, though the study could not establish the primary objective it set out to satisfy, it has raised some questions, had a stab at answering a couple others, and overall, has enchanted the medical romantic in me!
Reference

