
Linus Pauling, 1942
[Part 2 of 6 in a series investigating Pauling’s work on the serological properties of simple substances.]
The first four papers published by Linus Pauling and his Caltech colleagues on the serological properties of simple substances described general aspects of the precipitation reactions that occur between antibodies and antigens. This work was spurred by a fundamental conundrum: Pauling and many others knew that antibodies and antigens would react to form solid precipitates. However, because the chemical structures of these precipitates were, at the time, so difficult to determine, scientists had been unable to decipher crucial details about the antibodies and the antigens that combined to form them.
Pauling’s solution to this problem was to investigate the products of a reaction that utilized, in part, a chemical compound whose structure he already knew. The constituents of these products were a simple organic compound consisting of carbon, oxygen, and hydrogen, combined with one or more haptenic groups – small molecules that spur the formation of antibodies when coupled with a larger molecule. Employing this methodology would, Pauling felt, allow him to better approximate the make-up of the antibody, because the experiment now involved only one unknown structure.
In order to run the experiments, Pauling set up a standard protocol for preparing the compounds that he needed. Each experiment required three types of compounds: simple antigens used in the precipitation reactions; immunizing antigens used to create antibodies; and antisera, which are liquids containing antibodies formed through the coagulation of blood. Pauling used this method for all of his serological reaction experiments.
Pauling and his collaborators obtained the antisera by injecting rabbits (some of them housed in Pauling’s yard and cared for by his children) with immunizing antigens. The rabbits then produced antibodies to combine with and neutralize the immunizing antigens. Once the last injection was carried out, the scientists drew blood from the rabbits, allowed it to clot, and collected the antiserum.
The reactants for Pauling’s experiments – immunizing antigens and simple antigens – were either purchased or prepared by Pauling and his collaborators, typically the graduate students.
For each precipitation test, equal portions of antiserum and a saline solution containing a simple antigen were mixed together. Typically, four to six different concentrations of antigen were used. The mixtures stood at room temperature for one hour, then were refrigerated overnight. The next day, a centrifuge was used to separate out the precipitates, which were then washed with saline solution and analyzed. Pauling’s method of analysis involved measurements of nitrogen, arsenic, carbon, and hydrogen. From there, the amount of a given antibody in the precipitate was determined using the nitrogen measurements.
The initial set of experiments used twenty-seven different compounds as the antigen, each containing between one and four haptenic groups. All of the polyhaptenic substances – those that had more than one haptenic group per molecule – formed precipitates, but none of the monohaptenic substances did. This finding supported the framework theory, devised by the British chemist John Marrack in 1934, that postulated that multivalent antibody molecules could combine with polyhaptenic molecules to form large aggregates, which would become precipitates. On the other hand, Marrack suggested, if multivalent antibody molecules combined with monohaptenic molecules, only small complexes would form and these would not precipitate.
Pauling summarized this work in a set of four papers that were published in the December 1942 issue of the Journal of the American Chemical Society.

John Richardson Marrack
Pauling’s first article, “Precipitation Reactions between Antibodies and Substances Containing Two or More Haptenic Groups,” served primarily to provide support for Marrack’s framework theory. Eight years before, Marrack had stated that antibodies were multivalent; in other words, they can bond to more than one antigen molecule. In order for them to bind in this way, the molecules must be properly oriented such that the binding sites fit together. This causes the formation of a lattice-like structure which grows until it is too large to stay in solution and precipitates out.
As noted above, Pauling’s experiments found that “simple antigens containing two or more haptenic groups per molecule were found to give precipitates with the antisera, whereas the seven monohaptenic substances failed to precipitate,” a discovery that confirmed the validity of the Marrack-Heidelberger framework, or lattice theory.
The second paper in this installment was titled “The effects of changed conditions and of added haptens on precipitation reactions of polyhaptenic simple substances.” The alterations to conditions that were tested by Pauling included allowing the mixture to rest longer, changing its temperature, and altering its pH. Having confirmed his own belief, in Paper I, that antibodies are multivalent, Pauling used Paper II to first note his assumption – and provide evidence for – bivalence.
In addition, Pauling used this paper to publish an equation that could be employed to find the amount of a precipitated compound in a given solution based on solubility, equilibrium constant, and total amount of hapten. Notably, the equation led Pauling to deduce “that in each case the maximum amount of precipitate is produced by an amount of antigen approximately equal to the amount of antibody,” an idea that unfolded more fully in the following paper.
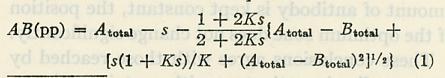
The equation published by Pauling in Paper II.
Paper III, “The composition of precipitates of antibodies and polyhaptenic simple substances; the valence of antibodies,” further explores the supposition of bivalence through an examination of the ratio of antibody to antigen in precipitates.
While the bulk of Pauling’s experiments focused on dihaptenic antigens, some used trihaptenic antigens, and others used tetrahaptenic antigens. Through careful analyses of the different precipitates that resulted, Pauling was able to determine that the ratio of antibody to antigen in any given precipitate was approximately 1:1.
This finding suggested that most antigens could have only two antibody molecules attach to them, even if they possessed more than two haptenic groups, since the antibody molecules were relatively large and interfered with one another’s attachment. Pauling also used the one-to-one ratio to conclude that most antibody molecules possess two binding sites. The major development of this paper – the near one-to-one ratio – was “taken to indicate bivalence of most of the antibody molecules.”
The last paper of the first installment, Paper IV, reported the results of initial experiments on the inhibition of precipitation in the presence of hapten. Pauling and his colleagues had tested precipitate inhibition in three basic ways: by altering temperature, by augmenting the amounts of hapten present in their mixtures, and by isolating the effects of twenty-four specific haptens. These experiments found that adding haptens to a mixture of antibodies and antigens inhibited the precipitation of the antibody-antigen complex.
Furthermore, Pauling concluded that the structure of the haptens correlated with their inhibition power and detailed the relative values of each hapten’s bond strength. He then used the hapten inhibition data from these experiments to update his earlier equation for finding the amount of antibody precipitated.
Next week, we’ll examine eight more papers that Pauling published on the topic over the next three years and explore the ways in which this body of research evolved and expanded during that time.
Advertisements