 Linus Pauling, Max Delbrück and Max Perutz at the American Chemical Society centennial meeting, New York. April 6, 1976.
Linus Pauling, Max Delbrück and Max Perutz at the American Chemical Society centennial meeting, New York. April 6, 1976.In 1940 Linus Pauling, along with colleague Max Delbrück, authored a three-page article that was published in the July issue of the journal Science. The length of the article was shorter than typical for Pauling, but what made it even more unusual was that it was not about Pauling’s findings. Instead, the piece served as a critique of a different article published earlier that year by a German scientist, Pascual Jordan.
In it, Jordan argued that when like molecules bonded, they were attracted more strongly than when dissimilar molecules bonded. Jordan believed that this stronger attraction of like molecules conferred special properties to these bonds, especially when they occurred in living cells. Pauling and Delbrück totally disagreed with this idea. Instead, the duo believed that it was a molecule’s complementary nature that conferred stability, an idea in opposition to Jordan’s concept of similarity.
In the two decades preceding these papers, chemists had come to look at their field in different ways, due mainly to advancements in quantum mechanics. This was certainly true for Pauling, who rapidly developed a reputation for using these new ideas to solve old problems. One line that he did not cross however, was the application of quantum mechanics to help “solve” topics that were already well understood and not in conflict.
For Pauling, one such instance was the basis of molecular attraction, and how that attraction created stability in a newly formed molecule. This idea, however, was something that other scientists found worth examining; Pascual Jordan in particular. Accordingly, and armed with a new set of quantum mechanical theories, Jordan set about attacking a question that others, including Pauling, believed not in need of answering.
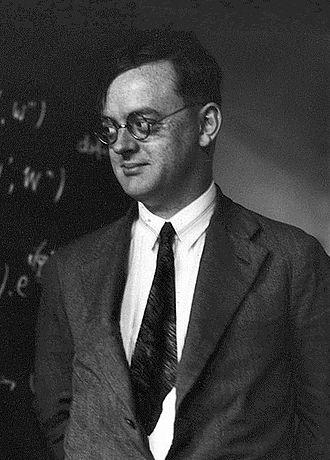
Pascual Jordan was born in Germany in 1902 of Spanish lineage. Though initially interested in the arts, Jordan studied math and physics in school, completing his physics Ph.D. in 1924. His ideas at this time were novel, with no less a figure than Albert Einstein taking note of his dissertation. But Einstein did not agree with certain of the hypotheses that Jordan was putting forth, many of which used quantum mechanics to consider the photon nature of light. While Einstein felt that there wasn’t necessarily anything wrong with Jordan’s ideas, he did not agree with the logic that informed them, and wrote missives in opposition.
But others supported Jordan’s work and, soon after graduation, he began working with a circle of colleagues that included Werner Heisenberg. During this time, Jordan became one of the biggest proponents of quantum mechanics and, along with Heisenberg, helped to unlock many of its secrets. Jordan was also a member of the Nazi party, joining when Germany entered World War II and remaining so until at least the end of the war. Nonetheless, Jordan helped to develop key theories in physics and math which are foundational to the fields today.
Though Jordan’s legacy today is marred by his political positions, when he wrote his 1940 paper about the attraction of molecules in biological cells, he did so from a position of authority. As noted, the foundation of the paper is the idea that identical molecules are attracted to one another in a special way that does not exist for dissimilar molecules and that, because of this, the bonds formed in molecules are more stable than is the case with other bonds. Jordan’s hypothesis, if true, would have been groundbreaking and consequential for all sorts of bonds, especially those in living cells.
Understandably, the paper created a lot of commotion when it was published. Pauling, who at that point was also an authority on quantum mechanics and resonance theory, was no doubt among those surprised by Jordan’s proposition. After reading it though, he immediately saw its flaws. In it, Jordan himself admitted some doubt that resonance could work in the manner that he was suggesting, and Pauling was sure that the ideas were wrong. Wishing to publish a rejoinder, Pauling began looking for a co-author whose expertise centered around bonds in living cells, and Max Delbrück was just such a figure.
Like Jordan, Delbrück was born in Germany in 1906. Interested in the stars, Delbrück began his studies in astrophysics, but changed directions upon meeting a physical chemist, Karl Bonhoeffer, who was eight years his elder. Fascinated by Bonhoeffer, Delbrück switched to physical chemistry in a ploy to become his friend, a tactic that ultimately worked well. The timing of the switch was also fortuitous as Delbrück entered the field at the beginning of the quantum revolution. After graduation, Delbrück studied all over Europe with scientists included Wolfgang Pauli and Niels Bohr. He eventually spent a few years at the California Institute of Technology on a Rockefeller Foundation fellowship, during which time he met Pauling and co-authored the 1940 paper. After leaving Caltech, Delbrück focused his research on bacteriophages and eventually won the 1969 Nobel Prize in Physiology or Medicine for this work.
Even though Delbrück’s Nobel honor was nearly thirty years down the road, by 1940 he was already well-versed on the ways that living cells operated, making him a formidable writing companion. In their paper, Pauling and Delbrück argued that Jordan’s fundamental idea could not be correct because the stability of a molecule was conferred by the complementarity its components, not their similarity. By way of explanation, the duo first put forth the understanding that a stable molecule is one in which molecular distances are relatively short. This is a circumstance, they argued, that can best be achieved when complementary forces are working together, such as positive ions attracting negative ions. In other words, in a bonding pair “the two molecules must have complementary surfaces, like die and coin.” The like molecules that Jordan was advocating for were not complementary by definition; rather, they were identical, or close to it. Pauling and Delbrück acknowledged that “the case might occur in which the two complementary structures happen to be identical” but still their stability “would be due to their complementariness rather than their identity.”
Even though Pauling and Delbrück’s article was quite short, its message was clear: Jordan was plainly wrong. As they wrote, “We have reached the conclusion that the theory can not be applied in the ways indicated by him [Jordan], and his explanations of biological phenomena on this basis can not be accepted.” In short order, the scientific mainstream came to agree with their point of view, and Jordan’s ideas soon faded away.
