Long time no post! Other writing commitments—and the death of my laptop, containing two half-written posts—have conspired to keep me from getting any blogging done for the past couple of months, not to mention that being a postdoc in the US is somewhat more intense than it was in the UK. I’ll try and get back on some kind of semi-regular posting schedule again, even if it's just once or twice a month for the time being. Thanks for your patience! —BRSM
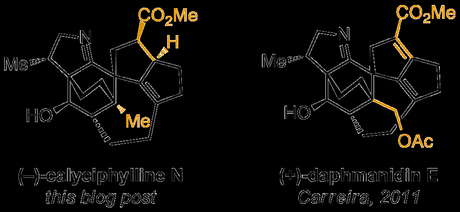
Total Synthesis of (−)-Calyciphylline N
A. B. Smith, III et al., J. Am. Chem. Soc., 2013, ASAP [PDF][SI][GROUP]
DOI: 10.1021/ja411539w
If you’ve read more than a couple of posts on this site, you’ll have probably noticed by now that I’ve got quite a soft spot for chemical history and syntheses of so-called 'classic' targets. Aside from the fun of comparing how the techniques for actually making molecules have evolved—and marvelling at some of the dangerous reactions people used to do—it’s great to examine targets that have been made a number of times and compare the routes that different chemists chose. If I had a bit more time, I’d write a lot more blog posts in this vein. Or a book.[1]
In fact, so similar is the structure of calyciphylline N—whose total synthesis was published by Amos Smith last week—to that of daphmanidin E, which Eric Carreira conquered back in 2011, that I immediately found myself wanting to look through my old blog post and compare the two approaches. I'm not going to write this blog post up as a head-to-head comparison of the two, mostly because they're both heroic endeavours in their own rights, and hence such a post would be quite unwieldy—and certainly not casual holiday reading—but I'd encourage you to take a look for yourself.
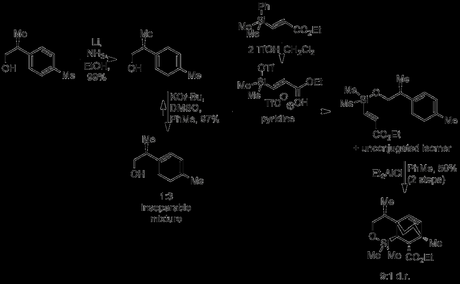
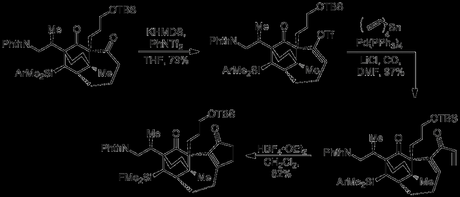
Smith’s approach began with a reaction that fortunately tends to perform better on a large scale: the Birch reduction.[2] Thus, the starting enantioenriched alcohol was transformed into the expected 1,4-diene, which was then isomerised into the conjugated 1,3-diene under basic conditions. This was then attached via its alcohol functionality and a silicon tether to ethyl acrylate using some interesting silicon chemistry and a rather unusual salt. When the coupled product was treated with a Lewis acid, an intramolecular Diels–Alder reaction between the diene and the tethered acrylate occurred, neatly using the molecule’s existing stereocentre to set four more and producing the tricyclic cycloadduct with good d.r. and in reasonable yield. Oh, and the reaction is reported on an impressive 90 g scale, no doubt generating way more aluminum by-products than I'd care to deal with in a workup!
The cycloadduct ester was reduced to the primary alcohol that was then homologated by conversion to the iodide, displacement with cyanide and reduction of the nitrile to give the chain-extended primary alcohol. Epoxidation of the cyclohexene (from the top face), followed by opening of the newly formed epoxide by the nearby alcohol and oxidation of the secondary alcohol generated. Now that it had performed its role in assisting in oxidation of the bicyclic core, the primary alcohol was regenerated by cleavage of the pyran ring with samarium iodide. TBS protection of the alcohol was then followed by α-Acetylation of the ketone (from the β-face); a reaction that turned out to be surprisingly challenging. Acetyl chloride and other electrophiles failed, but reaction of the enolate with acetaldehyde followed by oxidation with Dess–Martin periodinane gave the product in excellent yield. As my PhD advisor used to say: "when the going gets tough – don’t eliminate, add!"
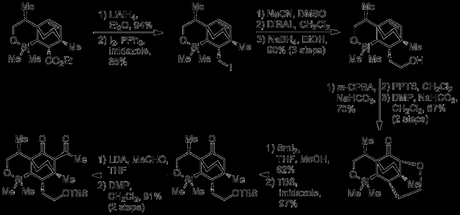
Palladium-mediated allylation of the 1,3-diketone was then followed by deprotection of the nearby primary alcohol, which was then converted to the iodide and used to alkylate the methyl ketone, forming the 3rd ring of the natural product. The allyl group was hydroborated and the resulting alcohol was protected. Now that the siloxane had done its job in tying up the hydroxyl group from the starting material, treatment with 4-methoxyphenyllithium ejected the alcohol, opening up the ring but leaving the silicon group in the molecule for later use. Finally, the newly liberated primary alcohol was converted to the protected amine by Mitsunobu reaction with phthalimide.
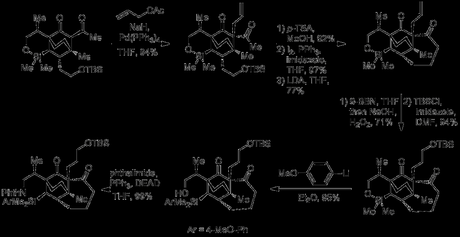
Next, the group used a rather usual method to introduce the first of the molecule's 5-membered rings, initially in the form of a cyclopentenone. Thus, the cycloheptanone was converted to the corresponding vinyl triflate, which was then subjected to a carbonylative Stille reaction with tetravinyltin. Treatment of the resulting dienone with tetrafluoroboric acid diethyl ether complex then effected a Nazarov cyclisation, along with simultaneous conversion of the aryl silane to the silyl fluoride, activating it for removal.
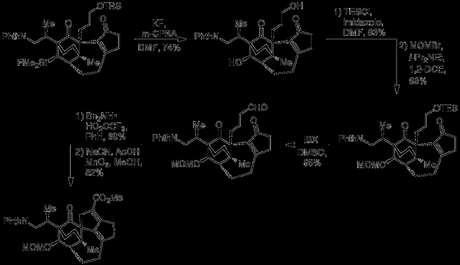
Now, having successfully served as a tether in the Diels–Alder reaction at the start of the route, then as a protecting group for the starting material alcohol for some 18 steps the silyl group could now perform its final role: as a latent alcohol. Thus, treatment with KF and m-CPBA successfully effected Flemming–Tamao oxidation to the secondary alcohol, with complete retention of stereochemistry. The primary alcohol was selectively protected using TESCl and imidazole in DMF and the newly introduced secondary alcohol was then capped with a MOM group.[3] The TES protected primary alcohol was then oxidised directly to the aldehyde by treatment with IBX and this was condensed with the nearby ketone using some conditions developed by Carreira en route to Daphmanidin E. Corey–Ganem–Gillman oxidation of the enal successfully converted it in one step to the methyl ester found in the natural product.
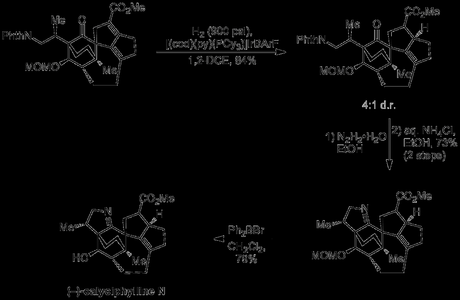
Next came the selective reduction of just the α,β-unsaturation of the dienoate, a reaction that I would have been very reluctant to rely on this late in a total synthesis. I suspect the Smith group began to regret doing so as standard, non-standard and downright wacky conditions failed, but they eventually lucked out, obtaining a good yield and d.r. with a modified version of Crabtree’s catalyst, where the usual PF6− anion had been switched for the much larger and more expensive BArF. With the end now in sight, the phthalimide protecting group was removed under classic Robinson–Ing–Manske conditions, a touch of acid was used to encourage its condensation with the cyclohexenone , and the MOM protecting group removed to give the natural product.
Looking back at the blog archives, it seems that this is actually the first Amos Smith synthesis that I’ve covered in detail, which surprises me somewhat given the group's long involvement in the field. That said—in my mind at least—I get the impression that in recent years the group has published a lot on its ARC methodology at the expense of natural products synthesis (e.g. this Angewandte paper from a couple of weeks ago). Still, there's no doubt that the total synthesis of calyciphylline N is an impressive achievement—especially for a single person doing the labwork. While the route maybe lacks a single standout awesome step or strategy, I did enjoy the clever use of silicon chemistry and the late stage carbonylative Stille-Nazarov sequence, and congratulate the authors on a job well done!
Addenda
1. Obviously, I try when I can to compare newly published syntheses to those that have gone before, as it’s one of the few ways that I can add value to what’s in the papers themselves, but I rarely write head-to-head comparisons of synthetic routes to the same target as it tends to make posts very long, and it takes ages. If you're interested in such things, I have done comparison posts for syntheses of Taxol (all of them!), prostaglandin PGF2α (Aggarwal vs. Woodward), the welwitindolinones (Rawal vs. Garg) and kibdelone C (Porco vs. Reddy), but over 2 years of blogging that makes them pretty uncommon.
2. My personal record for a Birch reduction is 100 gram scale, although that was from a starting material that was actually commercially available (an anisole derivative). Conversely, Smith had to make his benzyl alcohol substrate from p-tolylacetic acid using an Evans-type asymmetric alkylation (3 steps; ~60% overall yield), and hence only reports the transformation on 28 grams.
3. I think it’s a bit weird that the group decided to use MOMBr for this transformation, as there are very, very few cases when MOMCl won’t cut it.
