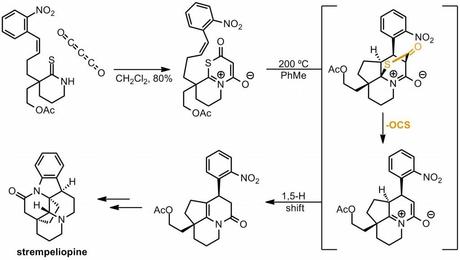
Perhaps Organic and Biomolecular Chemistry isn't a journal well known for its reviews, however I recently enjoyed reading this rather unusual perspective by Jason Chen on "Gas Extrusion in Natural Products Total Synthesis". All the classics are there: the retro [4 + 1] cycloaddition of sulfolanes to generate dienes (and the related Ramberg–Bäcklund reaction), the Boger-style 1,2,4,5-tetrazine [4 + 2]-retro-[4 + 2] method for the synthesis of aromatic rings and many more unusual ways to lose nitrogen besides. There are also some much rarer reactions, including an example from Padwa's synthesis of stempeliopine where addition of carbon suboxide (O=C=C=C=O) to a thioamide is followed by a cascade that eventually spits out carbonyl sulfide (OCS). I bet that's a synthesis that makes you unpopular in the lab[1]
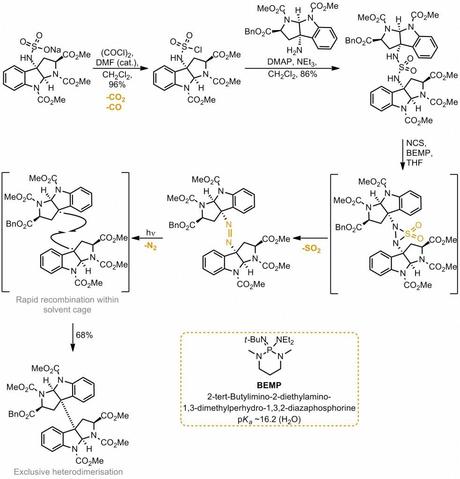
Probably the winner for the gassiest sequence described is from a Movassaghi paper that I remember from last year. Although the reaction hasn't actually been used to make a natural product yet, it looks like a useful way to access hetero cyclotryptamine natural products, of which there are many. The idea is to synthesise an unsymmetrical sulfamide, where the two amines are connected to the two units to be linked. Oxidation of this with NCS then generated a thiadiaziridine dioxide in some bizarre Aza-Myers-Ramberg-Bäcklund-type reaction. This then lost sulfur dioxide as expected to give a diazene that was decomposed photolytically with loss of nitrogen to give a pair of radicals that recombined quickly. Amazingly this recombination occurred sufficiently to prevent the formation of homodimeric products. Neat reaction - I love the fact that the linker is completely traceless!
Gas extruding reactions are more important that might be imagined at first (especially for the construction of strained systems), and it's nice to see a review dedicated to them.
Etc.
1. Carbon suboxide apparently smells (surprisingly?) awful . From an early review on its chemistry:
"The physical properties of carbon suboxide are of considerable interest. It is a gas under ordinary conditions, having an unbearable odor like acrolein and mustard oil. In small amounts it acts as a lachrymator; in high concentrations it attacks the eyes, nose and breathing organs, giving a feeling of suffocation."
There are lots of ways to make it, some of which are more appealing than others. Padwa generates it from dibromomalonoyl dichloride and zinc in ether at room temperature, which sounds quite sensible. Unfortunately, the OCS by-product also smells pretty bad. It's a little known fact that pure carbon disulfide has a pleasant smell redolent of diethyl ether (according to the Merck index; I used to have a CS2 still and I never experienced this). The reason it's so unpleasant to work with are traces of OCS (and thiols) from its manufacture. According to Wolf and Amarego these can be removed by washing with aqueous KMnO4 solution, followed by mercury. Or not.
