For a related post on things to check before publishing your controversial results to avoid potential humiliation, see my old piece on 'metal-free' reactions.
It seems to me that people still talk a great deal about so-called 'non-thermal' effects in microwave reactions; i.e. some kind of mysterious rate acceleration that occurs from excitation (or even stabilisation) of particular bonds or intermediates directly, in a fashion distinct from simple macroscopic heating of the reaction mixture. One of the most prominent critics of those who claim to observe these phenomena is Austrian chemist Oliver Kappe, who's been debunking claims of non-thermal effects for at least a decade and he's just written an excellent Essay in Angewandte Chemie on the subject. Now, few people would argue that a lot of reactions work better in the microwave, but this can often be explained by rapid internal heating or increased pressure; that is, by thermal effects, however dramatic. An example from Kappe's essay illustrates the incredible magnitude of rate improvement that's sometimes observed:[1]
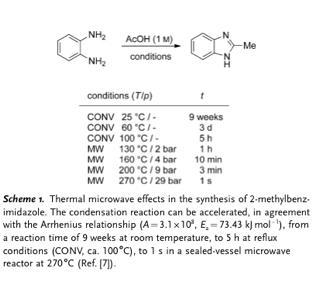
From Angew. Chem. Int. Ed. 2012, 51, 2.
Proponents of non-thermal effects (or those playing devil's advocate) are probably now thinking that surely if microwave-heated reactions were run in open vessels then it would be possible to separate any high pressure/superheating related effects and allow a straight comparison with conventional heating. Unfortunately, it turns out that it's not really that easy to compare microwave and conventional heating on equal terms. The reason for this is that for a fair and scientifically valid comparison the reaction heating and cooling profiles must be exactly the same, with even (or identically uneven) heating throughout. And that's not easy for viscous or non-homogenous reactions run in high dielectric solvents. This problem is compounded by the fact that most synthetic chemists tend to measure the temperature of microwave reactions using the surface IR sensors that most commercial microwave reactors come equipped with, despite the fact that such technology is notoriously unreliable, as Kappe points out:
"in many cases where specific/non-thermal microwave effects have been claimed, a subsequent careful reinvestigation has revealed that experimental artifacts mainly connected to erroneous temperature measurements and agitation/stirring of the reaction mixture were responsible for the originally observed phenomena. Importantly, during the past few years it has been recognized that the general practice of using external IR temperature sensors in microwave reactors that record the outer surface temperature of the reaction vessel— not the internal reaction temperature — is highly problematic when reliable on-line temperature data are required, as in kinetic experiments connected to the investigation of microwave effects. Internal, fast-responding fiber-optic (FO) temperature probes are far better suited to accurately monitor the actual reaction temperature during the microwave irradiation process, in particular for strongly microwave-absorbing and/or viscous reaction mixtures."
Kappe then quite pointedly states that:
"Based on our experience in studying microwave effects during the past decade, we were rather confident that non-thermal microwave effects in organic chemistry do not exist. In our hands, when reinvestigated using proper control of the process parameters (in particular temperature and stirring efficiency), all previously reported non-thermal microwave effects turned out to be purely thermal in nature, and ultimately the consequence of erroneous temperature measurement. For specific microwave effects, the situation is not as clear cut, although also in this case we have been largely unsuccessful in demonstrating any genuine and synthetically relevant effects, for example as a result of selective catalyst heating phenomena, or the elimination of wall effects."
At this point, in a manner that reminds me a little of Ben Goldacre, he proceeds to pick a few recent examples from the literature and debunk the authors' claims of non-thermal microwave effects. If you have access to the article, I'd definitely recommend that you read it, as the language is quite strongly worded for a scientific paper, and it's easy to imagine him grinding his teeth in parts. The first example he gives is an unusual Friedel-Crafts alkylation reported by Dudley and co-workers at the start of this year (Chem. Sci., 2012, 3, 1240) and described later in a Chemistry World article under the rather weighted title of Magical Microwaves.[2] As you can see, it appears that the reaction does work better in the microwave, even at the same temperature:
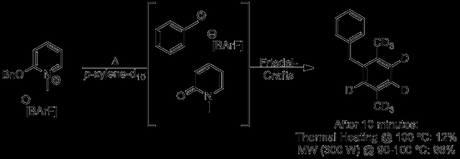
And here's the group's findings again, in a graphical comparison between thermal and microwave heating:
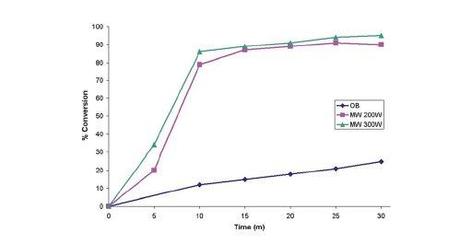
From Chem. Sci., 3, 1240.
Actually, it seems that Dudley and co-workers were quite careful, using the same volume reaction vessels for both runs, employing careful magnetic stirring and ensuring as much similarity as possible between the oil bath and microwave heated reactions. Unfortunately, despite its widely known limitations,[3] the group chose to rely on (calibrated) IR sensors to monitor the temperature of the microwave reactions, arguing that in this case the low viscosity, well-stirred, homogenous nature of their reaction medium would allow accurate measurements to be taken. Although that seems like a pretty good assumption, in their position might you not be tempted to run some control experiments with a fibre optic temperature probe, before publishing your controversial paper in a high impact and widely read journal loaded with phrases like 'clear benefit', 'rational design' and 'new paradigm'? Y'know, given that this would essentially be the first finding of its type? Well, don't worry because Kappe's done it for you:
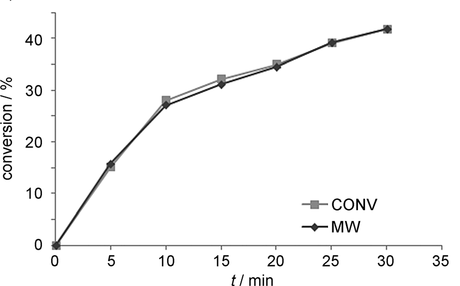
From Angew. Chem. Int. Ed. 2012, 51, 2.
Ouch! Well, hopefully no-one will notice. I get the impression that Kappe was even more appalled at the second example he gives, which deals with some recently reported (ACS Comb. Sci., 2011, 13, 2) conditions optimised for the formation of a specific hydrazone:[4]
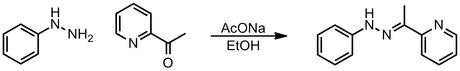
In this paper, the authors performed this reaction at reflux in an open, microwave heated vessel while simultaneously cooling the reaction mixture with compressed air to around 80 ºC. This reportedly gave a yield of 98%, a marked improvement over the 50% obtained in the absence of cooling air, or the <15% obtained for using a sealed vessel at 130 ºC. If you're interested in this technique of "simultaneous cooling", I suggest you the ACS Comb. Sci. paper, as I don't understand the reasons given for it all too well.[5] The authors make it sound very important, though, as it allegedly prevents bad things like "microwave overheating" and "decomposition". However, it does seem odd to me that such an elaborate setup is required just to make a simple hydrazone, as this is a reaction that's so reliable and general that it's used as a test for carbonyl groups in unknown compounds. Indeed, Kappe notes with quite tangible frustration that:
"In repeating the transformation shown in Scheme 3 using the exact same reaction stoichiometry and reagent concentrations as given in the original report, we noticed that hydrazine hydrochloride 3 reacts essentially spontaneously with ketone 4 at room temperature. Stirring the reaction mixture for 5 min at room temperature provided hydrazone 5, which precipitated in analytical purity, in 85% yield. Apparently, there is no need to heat this reaction. Nonetheless, we additionally performed a variety of different heating experiments (40–130 ºC) using either oil-bath or microwave heating in closed or open vessels applying proper internal FO temperature-sensing technology. In essence, the results were always identical: the desired hydrazone 5 was obtained in roughly 85% yield within 5 min. We were not able to detect any decomposition of the hydrazone product at elevated temperature. It is therefore apparent that there is no special microwave effect of any kind involved in this chemistry, and that the use of open-vessel microwave processing or applying simultaneous cooling does not lead to any apparent advantages."
So, in conclusion: there is no magic, don't believe IR sensors and don't forget that no clear evidence of a provable (let alone useful) non-thermal microwave effect has ever been reported. Make sure you run all those pesky little control experiments and remember that extraordinary claims require extraordinary evidence.
Etc.
1. You can read a lot more about the theory of micrwave chemistry, although with a lot more well chosen examples in Kappe's Tutorial Review from a few years back (Chem. Soc. Rev. , 2008, 37 , 1127).
2. Interestingly, Chemistry World has also run a few stories supporting Kappe's viewpoint including Microwaving Myths (not free) and the rather final sounding Microwave Effect Ruled Out (free).
3. I did most of my PhD work in an organic/inorganic lab next door to a polymer science lab. I remember chatting to one of the postdocs from the polymer group one day about borrowing some AIBN, of which they had, understandably, the largest stash of in the building. After a little casual conversation I found out they actually used the stuff (and far less stable initiators) on terrifying scale in the microwave. I remember asking about how safe this actually was to do, and was told that as long as fibre optics were used to allow decent temperature control that everything was find. I'd never heard of this technology at the time, but I remember being told horror stories of reactions where IR sensors gave temperature readings 50 ºC (or more) too low, causing massive overheating of their reactions with undesirable results. Since that conversation a few years back I've always had a healthy degree of mistrust for microwave temperatures obtained by IR measurements (i.e. most of them).
4. As an aside, 2-acetylpyridine has an extremely lingering although not entirely unpleasant smell which Aldrich gives the organoleptic oily. Although it's apparently an important contributor to the flavour of popcorn and beer I'd advise you to avoid it you can.
5. I'm not entirely sure they're all that scientific.
