
Killer whales are social animals that navigate all oceans and seas between the Arctic and Antarctica – they can be regarded eusocial since reproduction ceases around 40 years of age and menopausal females help care for offspring: like humans [13, 14]. Group cohesion in killer whales relies on a complex repertoire of vocalisations including clicks, whistles and calls. Sounds are instrumental for prey searching, orientation and communication. Foote [5] focused on calls, which are made up of series of discrete sounds that resemble squeaks, screams, and squawks to the human ear. It has been postulated that individuals learn to vocalise by imitation of peers of the same pod, and that only the base structure has a genetic, hence heritable, component [15]. Regardless, pods develop regional dialects. Those dialects, along with aspects of diet, genetics, morphology and behaviour, differentiate the three main lineages of killer whales (resident, transient and open sea) that might have been genetically isolated for ~ 150 to 700 thousand years and, potentially represent different taxa [16, 17]. The species might abandon the IUCN conservation category of ‘Data Deficient’ as soon its taxonomic uncertainty is resolved.Resident killer whales form matrilineal groups of 2 to 15 individuals (the matriarch and her offspring) – known as pods, in turn subdivided into subpods centred around grandmothers and great-grandmothers. The Southern Resident population is regarded as an acoustic clan comprising 3 pods currently numbering 81 individuals = 26 (J pod) + 19 (K pod) + 36 (L pod) (2013 survey), among whom the matriarch Granny is the oldest at 103 years! This clan feeds mainly on fish, and dwells in the coastal waters between British Columbia (Canada) and Washington State (USA), particularly south of Vancouver Island – nothing is known about where they spend the winter. The clan lost 20 % of its members between 1995 & 2001, and 13 more by 2013, and now faces the decline of its main prey: Chinook salmon (Oncorhynchus tshawytscha) [18]. The two pics show two sub pods of this clan swimming close to a whale-watching boat near Friday Harbour (San Juan Island) and a Chinese ship at Puget Sound (Seatle, USA). Photo credits: Marta Holt, NOAA/NMFS Northwest Fisheries Sciences Center.
Acoustic pollution has become a transnational issue, particularly in marine ecosystems [1] by virtue of the physical fact that sounds travel in water farther and faster than in air. In our noisy, modern world, many species are now forced to modify their vocal repertoire in response to noise. The pivotal social role that vocalisations play in all cetacean species makes these predators particularly vulnerable to this environmental problem.
–
Last night, an ambulance siren woke me, only seconds before the neighbour’s washing machine started spinning, and a good friend of mine rang from overseas. Gradually more and more people are living in societies plugged in to noisy mechanical and electronic devices 24 hours a day, 356 days a year.
Engine-powered vehicles are the main source of anthropogenic noise, and their numbers can grow even at a higher rate than the human population – so spreading not only diseases [2] but also decibels over a global network of traveling routes. In an ecological context, we refer to noise as a kind of sound (= energy wave detected by an auditory system) that is not considered a biologically meaningful cue by wildlife (including us) and might also cause physiological stress. Experts refer to ‘masking’ as those situations in which noise interferes the perception or emission of sounds that matter to the life history of species – a global concern in both terrestrial [3] and aquatic [4] ecosystems.
Andy Foote [5] has assessed the effect of vessel traffic on the vocal behavior of the three pods forming the Southern Resident population of killer whales (Orcinus orca¸ see video). He recorded calls from these cetaceans from a ship, and through an array of submarine microphones in Haro Straight, between San Juan Island (Washington State, USA) and Vancouver Island (British Columbia, Canada). Between the 1990s and the 2000s, local traffic density had multiplied by a factor of 5 and currently, > 20 whale-watching vessels follow these killer whales daily among an active fleet of > 70 commercial vessels. Foote compared call length through 35 hours of underwater killer whale recordings over three periods (1977-1981, 1989-1992, 2001-2003), each comprising situations in which the pods were exposed to both noisy and quiet environments. Over the study, call length varied between 0.3 and 2.0 seconds; while on average, L-pod calls were the shortest (0.6-0.8 seconds), and J-pod calls the longest (0.9-1.0 seconds).
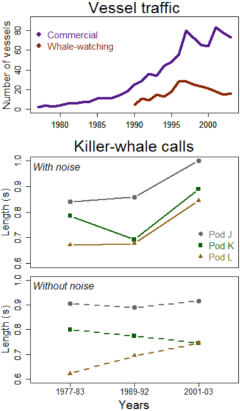
Shipping intensity (number of vessels) and underwater call duration in killer whales (in seconds) at Haro Straight. Call duration in killer whales (bottom graphs) peaked at the period of heaviest vessel traffic in the early 2000s (top graph). Vessel traffic was estimated from the Lime Kiln and the educational vessel Soundwatch Boater. Killer whale calls were digitalised in spectograms (graphs representing change in frequency over time), from which the most frequent call types were selected for further analyses (1, 3 and 6 in pods J, L and K, respectively – click on former hyperlinks for call samples from each pod). Call types are strongly stereotyped, and their classification can be done through published catalogues.
Most importantly, Foote found that call length differed between noisy and quiet environments only in the most recent period (2001-2003) when the total number of vessels had peaked at Haro. He postulated that noise levels might have surpassed a threshold above which killer-whale vocalisations are masked, and these animals are lengthening their calls to compensate for masking. In a parallel study, call amplitude in the Southern Resident population also correlated positively with increasing vessel noise and number [6].
Acoustic impacts that modify vocal repertoires are one among a variety of physiological (e.g., change in hearing thresholds and damage to inner ear) and behavioural (e.g., displacement from or avoidance of native habitat, change in swimming, diving and surfacing patterns) responses to noise that have been described or hypothesised across cetacean species [7, 8], and marine mammals in general [9]. Straightforward as it may sound, ascertaining cause-effect relationships between noise and physiological stress is a tricky business in cetacean research (well, it is a challenge in virtually any field of research). For instance, despite the large number of strandings of beaked whales (Ziphiidae) suffering from lesions of the inner ear, following naval exercises using sonar devices, cause-effect evidence remains arguably tenuous [10, 11]. Weilgart [8] has (i) classified the main sources of noise at sea [i.e., shipping, sonar operations, seismic exploration for oil; see also 12], (ii) reviewed that the potential effects of noise on cetaceans vary according to species, populations, and the age and ongoing part of the life cycle of individuals, and (iii) flagged that those effects can also be indirect if, for instance, they affect prey behavior. She proposes several areas of research that require urgent tackling, which I summarise below:
- Technological development: engines emitting environmentally friendly noise are needed (e.g., shorter, directionally narrower and free of unnecessary frequencies). Industries should comply with regulations of passive acoustic monitoring (arrays of microphones with continual noise recording) and modulation of activities tailored to the life history of neighboring fauna.
- Legislation: companies and governments undertaking naval operations both in coastal and offshore areas should monitor and publish their levels of noise. Many companies worldwide are enforced by law to report emissions of pollutants such as CO2; likewise, noise should be given the rank of pollutant and managed accordingly.
- Research: investigations are challenged by the home ranges of many species which often spread over hundreds to thousands of kilometres. Establishing causality between noise and animal responses requires a thoughtful design of comparable noisy versus control (noise-free) areas (e.g., similar habitat type, population density), improvement of protocols for monitoring stress-indicator hormones (e.g., from faecal samples), quantification of synergisms (noise interactions with other impacts like chemical pollution or fishing) and development of research methods that do not add more noise to sea.
- Stranding networks: a global database of cetacean strandings and standardisation of necropsy protocols would allow global diagnoses of impacts (e.g., auditory damage). Such database could give way to retrospective analyses if accompanied with an additional, global database of outstanding noise events.
- Conservation: noise should be banned at animal congregation areas and concentrated where biodiversity is relatively low, and become another criterion for the designation and management of marine protected areas.
After writing this blog and monitoring myself through an entire day, I have become stunningly aware that I produce noise in a steady fashion as much as I produce other kinds of wastes in a full range of domestic and work situations – something else to pay careful attention to.
–
References
- Dotinga, H.M., and Oude Elferink, A.G. (2000). Acoustic pollution in oceans: the search for legal standards. Ocean Development and International Law 31, 151-182
- Tatem, A.J., Simon, I.H., and Rogers, D.J. (2006). Global traffic and disease vector dispersal. Proceedings of the National Academy of Sciences of the USA 103, 6242-6247
- Barber, J.R., Crooks, K.R., and Fristrup, K.M. (2010). The costs of chronic noise exposure for terrestrial organisms. Trends in Ecology and Evolution 25, 180-189
- Slabbekoorn, H., Bouton, N., van Opzeeland, I., Coers, A., ten Cate, C., and Popper, A.N. (2010). A noisy spring: the impact of globally rising underwater sound levels on fish. Trends in Ecology and Evolution 25, 419-427
- Foote, A.D., Osborne, R.W., and Hoelzel, A.R. (2004). Whale-call response to masking boat noise. Nature 428, 910-910
- Holt, M.M., Noren, D.P., Veirs, V., Emmons, C.K., and Veirs, S. (2009). Speaking up: killer whales (Orcinus orca) increase their call amplitude in response to vessel noise. Journal of the Accoustical Association of America 125, EL27-EL31
- Nowacek, D.P., Thorne, L.H., Johnston, D.W., and Tyack, P.L. (2007). Responses of cetaceans to anthropogenic noise. Mammal Review 37, 81-115
- Weilgart, L.S. (2007). The impacts of anthropogenic ocean noise on cetaceans and implications for management. Canadian Journal of Zoology 85, 1091-1116
- Weilgart, L.S. (2007). A brief review of known effects of noise on marine mammals. International Journal of Comparative Psychology 20, 159-168
- D’Amico, A., Gisiner, R.C., Ketten, D.R., Hammock, J.A., C., J., Tyack, P.L., and Mead, J. (2009). Beaked whale strandings and naval exercises. Aquatic Mammals 35, 452-472
- Bradshaw C.J.A., Evans, K., and Hindell, M.A. (2006). Mass cetacean strandings – a plea for empiricism. Conservation Biology 20, 584-586
- Hatch, L.T., and Wright, A.J. (2007). A brief review of anthropogenic sound in the oceans. International Journal of Comparative Psychology 20, 121-133
- Foster, K.R., and Ratnieks, F.L.W. (2005). A new eusocial vertebrate? Trends in Ecology and Evolution 20, 363-364
- McAuliffe, K., and Whitehead, H. (2005). Eusociality, menopause and information in matrilineal whales. Trends in Ecology and Evolution 20, 650
- Filatova, O.A., Deecke, V.B., Ford, J.K.B., Matkin, C.O., Barrett-Lennard, L.G., Guzeev, M.A., Burdin, A.M., and Hoyt, E. (2012). Call diversity in the North Pacific killer whale populations: implications for dialect evolution and population history. Animal Behaviour 83, 595-603
- Morin, P.A., Archer, F.I., Foote, A.D., Julia Vilstrup, J., Allen, E.E., Wade, P., Durban, J., Parsons, K., Pitman, R., Lewyn Li, L., et al. (2010). Complete mitochondrial genome phylogeographic analysis of killer whales (Orcinus orca) indicates multiple species. Genome Research 20, 908-916
- Riesch, R., Barrett-Lennard, L.G., Ellis, G.M., Ford, J.K.B., and Deecke, V.B. (2012). Cultural traditions and the evolution of reproductive isolation: ecological speciation in killer whales? Biological Journal of the Linnean Society 106, 1-17
- Ayres, K.L., Booth, R.K., Hempelmann, J.A., Koski, K.L., Emmons, C.K., Baird, R.W., Balcomb-Bartok, K., Hanson, M.B., Ford, M.J., and Wasser, S.K. (2012). Distinguishing the impacts of inadequate prey and vessel traffic on an endangered killer whale (Orcinus orca) population. PLoS One 7, e36842

