A bottle of formic acid and a graduated cylinder showing the appearance of formic acid as a liquid. Label on the bottle says (translated from Serbian): "Formic acid For destruction of Varoa and disinfection HCOOH - 85%. Dose: use data from literature, put above the hive".
Physicist Florian Nitze at the Umeå University, Sweden, has developed several new catalysts that improve the capacity of the fuel cells, making it possible to use relatively environmentally friendly formic acid in fuel cell powering your mobile phone or laptop.
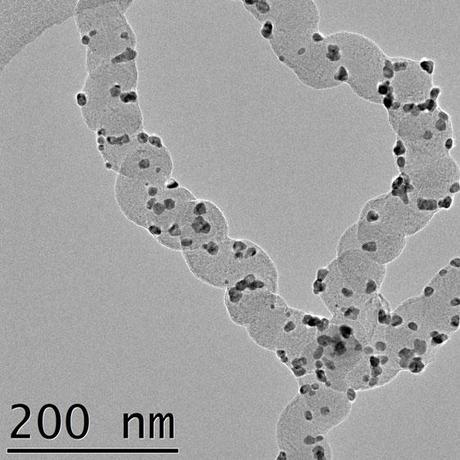
Helical formed carbon nanofiber. (Credit: Umeå University)
Fuel cells are different from batteries in that they require a constant source of fuel and oxygen to run. The technology is already commercially available but formic acid fuel cells still suffer from low power and lifetime.
The effect of a catalyst is to reduce the energy loss and to increase the rate of the chemical reactions, which leads to a higher efficiency in the fuel cell.
If for example hydrogen and oxygen (but equally valid for formic acid and oxygen) get in contact, they can burn and release a lot of energy. In this process hydrogen gives electrons to oxygen, it is oxidized whereas oxygen takes electrons from hydrogen, it is reduced.
The idea of a fuel cell is to separate these two reactions spatially into two separate reactions, namely oxidation and reduction. The energy that would have been released by burning can now be used as electric power if the two separated reactions are connected electrically. However, not all energy can be used; some energy is needed to keep the reaction running. Catalysts can lower this energy loss and speed up the reactions resulting in a higher efficiency of the fuel cell.
In his thesis, Florian Nitze describes new catalysts based on a combination of materials science and nanotechnology—engineering on an atomic scale.
“Especially catalysts of palladium nanoparticles attached to a unique helical formed carbon nanofibre proved to have a long lifetime and a very high potential to be used in formic acid fuel cells. The helical formed carbon nanofibre has a high electrical conductivity and a surface that is very easy to decorate with nanoparticles,” says Florian Nitze.
Several of the new catalysts that Florian Nitze have developed are based on palladium. It is a noble metal such as gold or platinum, but it is half as expensive.
Formic acid can be produced from renewable sources, i.e. wood, and is therefore a highly environmentally friendly alternative. Formic acid has low toxicity and is even used as a food additive. The concentrated acid is, however, corrosive to the skin.
“One of the major advantages over Li-ion batteries, which are dominating the battery market, is that the charging only takes seconds by simple refueling with formic acid,” says Florian Nitze.
Nitze, F., Mazurkiewicz, M., Malolepszy, A., Mikolajczuk, A., Kędzierzawski, P., Tai, C., Hu, G., Kurzydłowski, K., Stobinski, L., Borodzinski, A., & Wågberg, T. (2012). Synthesis of palladium nanoparticles decorated helical carbon nanofiber as highly active anodic catalyst for direct formic acid fuel cells Electrochimica Acta, 63, 323-328 DOI: 10.1016/j.electacta.2011.12.104
