I'd originally planned to do four of these posts, but it looks like I've run out of time so I'll be getting back to more cutting edge work (as soon as something exciting is published). Maybe I'll post the last one in March Mulch. Check out Mulvember 1: Penfulvin A and Mulvember 2: Echinopines A and B!
Okay, I suppose I should start off by acknowledging that Mulzer isn't the corresponding author on this one (instead it's Mulzer group postdoc Jürgen Ramharter), but it's still a nice piece of work so I'm including it anyway. The target itself is one of the perennially popular lycopodium alkaloids whose first member - lycopodium itself - was isolated way back in 1881. A number of classic syntheses of members of this family in the 1970s and 80s by famous alkaloid chemists such as Stork, Heathcock, Wiesner and Wenkert have set the bar pretty high, but work towards these targets continues to this day.[1] Particularly, the fawcettimine-type members of this family, to which lycoflexine belongs, have proved very popular in recent years with a new synthesis seemingly out every few months.
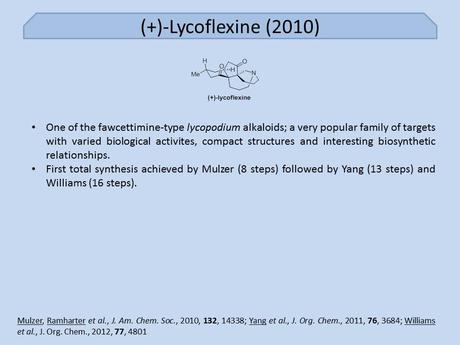
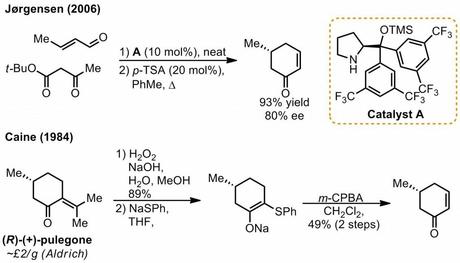
As Totally Synthetic remarked at the time, calling this work concise is a bit of an understatement as it took the group all of eight synthetic steps to complete the molecule, starting from a well-known chiral building block: (R)-5-methylcyclohex-2-enone. Although it’s good to be suspicious of ‘readily available’ chiral starting materials (lest they take 16 steps to prepare),[2] there’s no foul play here as it’s been shown that the required compound can be simply prepared by a one-pot organocatalysed reaction between t-butylacetoacetate and crotonaldehyde. However, it turns out that (according to the paper’s SI) the group actually used the slightly longer route developed by Caine, taking a dip in the chiral pool with (+)-pulegone, presumably for better ee or scalability:
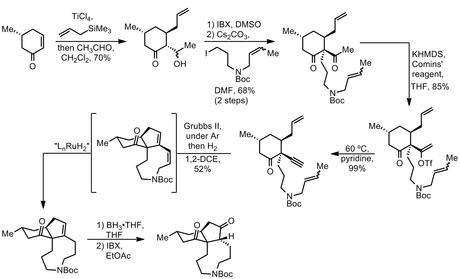
Taking this starting material, the group then performed a Hosomi-Sakurai-type 1,4-addition of allyltrimethylsilane to the enone, trapping the resulting titanium enolate with acetaldehyde. After oxidation with IBX in DMSO, the resulting 1,3-diketone was alkylated with the iodide shown, successfully setting the target’s single quaternary centre in good yield. Next came a transformation I hadn’t seen before – the conversion of a methyl ketone to the corresponding alkynyl by vinyl triflate formation followed by elimination. This is formally the ‘opposite’ of the more common conversion of alkynes to methyl ketones using mercury(ii) assisted hydration. Treatment of this alkyne with 20mol% of Grubbs' second generation catalyst under argon then gave the expected enynene metathesis product, but the reaction didn’t stop there. Inspired by a little-known Grubbs paper,[3] the group then changed the inert atmosphere to hydrogen, which lead to smooth, selective hydrogenation of the cis olefin to give the protected azonane in quite reasonable yield.[4] A final hydroboration-oxidation sequence then yielded the tricyclic diketone.
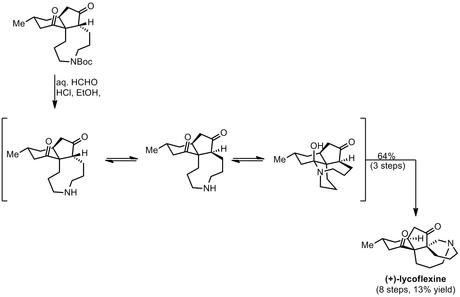
Finally, the planned deprotection/transannular-Mannich cascade occurred smoothly upon treatment with aqueous formaldehyde solution under acidic conditions in ethanol, delivering the natural product in great yield over three steps from the enynene metathesis product.
 By making excellent use of cascade sequences to achieve multiple operations in a single reaction vessel, the group have managed an extremely short and efficient synthesis of the target, which I can’t see being beaten any time soon. I particularly enjoyed the enynene metathesis/hydrogenation combination and the final cascade.[5] Like many Mulzer syntheses, this work both taught me a few things and showed me a route that I’d never have thought of, and can’t really improve on. I’ll miss seeing Mulzer papers in the literature as they always get me excited about synthesis. Johan Mulzer, if you’re reading this (and I kinda hope you’re not), enjoy retirement and thank you!
By making excellent use of cascade sequences to achieve multiple operations in a single reaction vessel, the group have managed an extremely short and efficient synthesis of the target, which I can’t see being beaten any time soon. I particularly enjoyed the enynene metathesis/hydrogenation combination and the final cascade.[5] Like many Mulzer syntheses, this work both taught me a few things and showed me a route that I’d never have thought of, and can’t really improve on. I’ll miss seeing Mulzer papers in the literature as they always get me excited about synthesis. Johan Mulzer, if you’re reading this (and I kinda hope you’re not), enjoy retirement and thank you!
Etc.
1. To give you an idea of how good some of this work is: most people probably noticed T. Hud.'s rather inflammatorily titled essay on the problems with modern organic chemistry over in Helvetica Chemica Acta. After Derek put this paper up for discussion over at In The Pipeline, Hudlicky himself waded into the comments to address questions from readers. When he was challenged by Martyn to name a recent synthesis he actually liked he replied:
"...look up Clayton Heathcock's lycopodine synthesis (Full paper in JACS, 1980s-1982?) I know, that is not recent, but a good example of excellent chemistry and honest reporting. Who gives a F*&^ about yields anyway? If our stuff is at all useful, the process guys will make it >90% in no time. By correctly performed optimization. And from that source, I will believe it!"
It's here, if you're curious. Or you could check out this nice Stoltz group meeting presentation on the subject.
2. I’m looking at you, Holton taxol synthesis.
3. When I say ‘little known’, I really mean ‘little known to me’; it has over 200 citations. If you’re interested see J. Am. Chem. Soc., 2001, 123, 11312.
4. Although the selectivity of this hydrogenation might seem a little non-obvious, it’s perfectly in line with the cis-disubsituted > trans-disubstituted > conjugated, trisubstituted trend reported in the original 2001 Grubbs paper. Another interesting feature of the hydrogenation conditions is that hydro-dehalogenation is reported to be very slow.
5. If you enjoy cascade reactions, there’s a large Chem. Rev. article on them out recently with lots of good problem session fodder.
