Although there have been a couple of interesting syntheses this week, I'm still very busy so I'm going to write about another Mulzer synthesis from my talk. See my previous post for the background to this tribute.
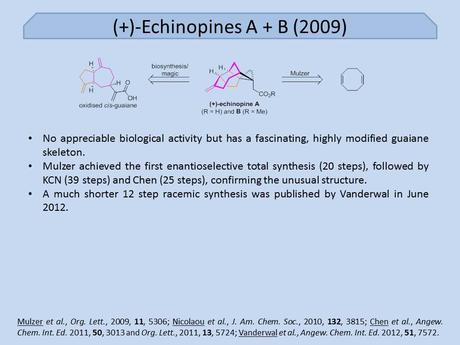
Since their fairly recent isolation in 2008 the echinopine sesquiterpenes have proved quite popular targets for total synthesis. In fact, four rather different total syntheses have been reported since their unusual and compact molecular architectures first graced the literature. The first of these was that of Johann Mulzer, published just a year after their isolation, in which both natural products were synthesised in near enantiopure form (starting from cyclooctadiene!) and their absolute configurations were confirmed for the first time.[1]
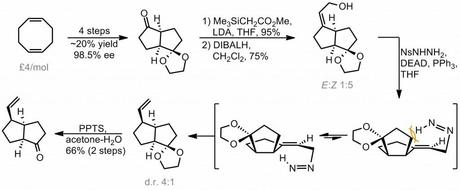
The route began, surprisingly, with cyclooctadiene, a quite uncommon starting material in natural products synthesis. This was tranformed via some known chemistry to an enantiopure bicyclooctanone in four steps, including a palladium mediated transannular cyclisation and resolution with a lipase. A Peterson olefination, followed by reduction of the newly installed ester with DIBALH then gave the allylic alcohol shown. Next, the group made clever use of Myers' reductive allylic transposition to introduce a new stereocentre with good diastereoselectivity under substrate control.[2]
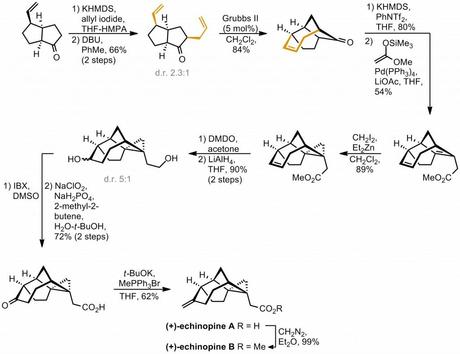
Alkylation of the ketone alpha-position was then carried out using KHMDS and allyl iodide, unfortunately occurring with rather poor diastereoselectivity. This problem could be partially rectified by epimerisation of the newly formed stereogenic centre by heating with DBU in toluene, but this still only lead to a (HPLC separable) ~2:1 mixture of diastereomers (in favour of the desired one). Better luck was obtained in the following RCM step which allowed closure of the seven membered ring in great yield with Grubbs' second generation catalyst. Considerable effort was expended in trying to combine these two reactions in a single pot, which sounds like a good idea as the RCM is only possible on the correct diastereomer so could be used to drive the epimerisation to completion, but unfortunately didn't work out in practice. The use of a more functionalised side chain than plain allyl also caused the RCM to fail. Pressing on, installation of the ester was carried out by conversion of the ketone to its vinyl triflate, followed by a Heck-type reaction with the silyl enol ether of methyl acetate. Next, regio- and stereo- selective cyclopropanation was carried out using that most directable of reactions: the (Furukawa-) Simmons-Smith.[3] A four step sequence was then used to introduce the exo-methylene group found in the natural product, beginning with epoxidation of the remaining olefin with DMDO under substrate control. It was then found that the epoxide from this reaction could be opened regioselectively by treatment with lithium aluminium hydride, which unsurprisingly also reduced the side-chain ester. A duo of oxidations was then used to convert the new secondary alcohol to the corresponding ketone and bump the primary alcohol back up to the carboxylic acid. Finally, treatment with a suitable Wittig reagent lead smoothly to the formation of echinopine A and this could be converted to echinopine B through simple esterification with diazomethane. Another synthesis complete!
Trivia
1. As I said when I wrote about the recent Vanderwal work in this area, I was surprised that Mulzer's paper ended up in Org. Lett. Heck, my last two Org. Lett. papers don't contain half as much work as this one! Furthermore, it takes Mulzer a mere 20 steps to the target from commercial materials, compared with Chen's 25, and KCN's 39, and the route is plenty clever, especially for the first synthesis of such an unusual target.
2. I think this is quite a cunning way of forming a vinyl group and a new stereocentre in a single step. If you can't remember how it works, I discussed it a few months back in a post I wrote on hydrazone chemistry.
3. There's a classic Dave Evans review on substrate directable transformations (Chem. Rev., 1993, 93, 1307). These were very important in the days before fancy things like asymmetric catalysis, when one had to use the existing stereocentres in a compounds to guide the introduction of further ones, and still see plenty of use. The review is worth reading just to see a very young Greg Fu failing at wearing a bow tie:

Bow Tie Fail.
But we digress.
