Like many research groups, the one I’m in does weekly literature talks so people get a bit of practice with powerpoint and public speaking. Because excessive freedom can be a bit daunting, although people are free to choose the topic of their own talk it has to fit in with a particular theme, which, at the moment, is living Germanic chemists. In this vein, last month I wrote and gave a talk on the life and work of Johann Mulzer. Now, as I've been a bit busy lately, and the literature has been a bit lacking in interesting total syntheses, I've decided to rehash my talk as a series of blog posts. On the upside, this should mean more posts for you guys and less hassle for me (as I've already drawn everything in ChemDraw). Also, although I didn't know this when I wrote the talk, it seems that Mulzer is finally winding down and I think he deserves a bit of send-off. I, for one, have learnt a lot from reading his papers over the past few years.

From a recent Angewandte paper.
Unfortunately, most of the syntheses that I covered in my talk are already pretty well known, and many of them have also already been covered on Totally Synthetic at one time or other. Still, if you missed somehow missed reading about them there or prefer my more rambling style then read on!

Incidentally, if you’re wondering what the German text on the slide is all about, it’s taken from the group website and is usually rendered (non-literally) in English as ‘no battle plan survives contact with the enemy’, something all chemists who have worked in total synthesis know well![1]
Mulzer’s career got off to a good start beginning with a PhD supervised by Rolf Huisgen (of 1,3-dipolar cycloaddition fame), followed up – like so many successful academics – with a postdoc with E. J. Corey. After hopping around Germany for a bit he eventually settled down in Vienna, where he’s been for the last 16 years. As 20 minutes is a rather short time to cover the career of a man with over 300 publications I decided to limit myself to just talking a bit about the work that Mulzer is probably best known for; total synthesis.
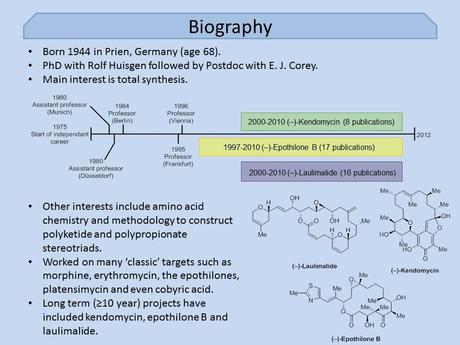
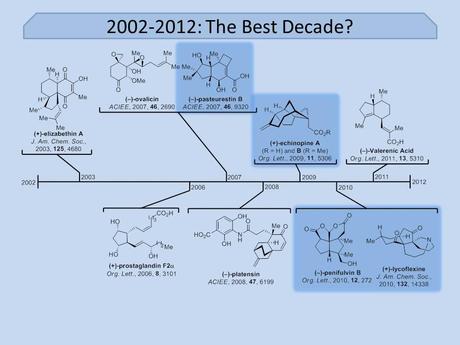
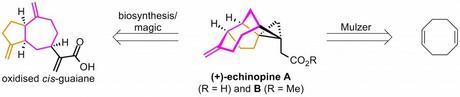
Unfortunately, as this didn’t narrow things down as much as I’d hoped, I decided to also exclude his long term work on the (larger) targets laulimalide, kendomycin and epothilone B and focus just on a few of his more recent syntheses of small molecules, which I think is what a lot of people from my generation will remember him for. I mean just look at these structures! Anyone who’s taken even a slight interest in the synthetic chemistry literature over the last few years should immediately recognise hot targets such as platensin, prostaglandin F2α, echinopine A and the lycopodium alkaloid lycoflexine. Clearly the Mulzer group weren't afraid of a little competition!
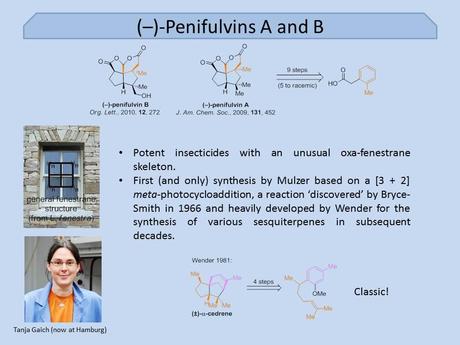
Because I’m not a particularly well organised person - and also for narrative purposes - rather than work in chronological order I decided to cover penifulvin A first of all. This synthesis, which you might have seen in Paul's Chemistry World column, made use of a reaction I love: the [3 + 2] meta-photocycloaddition. In fact, I like it so much that it was the subject of my post in last year's Favourite Reaction Chemistry Carnival:
I will now tell you a little bit about a much less well known example - the [3 + 2] meta-photocycloaddition reaction of arenes and alkenes (which unfortunately doesn't have a name, as far as I'm aware). Before I show you what it is, let me explain why I've picked it. I still remember the first time I encountered alkene metathesis as an undergraduate, and the feeling of awe that we chemists could have access to such an apparently magical transformation. I mean, to one beginning their chemical studies the ability to chop and change carbon-carbon double bonds without any activating groups nearby seems completely fantastic. Similarly, I was totally shocked when I saw my first [3 + 2] arene-alkene cycloaddition a few years later in Mulzer's 2009 total synthesis of penifulvin A. It's just such an improbable looking reaction in which a benzene ring can be coaxed into forming two bonds simultaneously to a nearby alkene, also generating a cyclopropane and some of the hardest to draw intermediates you'll ever meet.
In fact, I encountered this application just a few months into my PhD studies and was amazed to see such a strange transformation used in such a clever way,[2] by a man I’d never even heard of! I remember thinking at the time that I should remember this Mulzer person![3]
As they're quite similar, I only covered the group's enantioselective synthesis of penifulvin A in my talk (they also made B and C), which means that's all you're getting here.[4] The route began with the synthesis of the enantioenriched alcohol required for the meta-photocycloaddition step. This was done by Myers' alkylation of the commercially available starting acid with homoprenyl bromide, followed by reduction of the amide with LiNH2BH3 (generated in situ from LDA and commercial NH3�•BH3).[5] The alcohol was then irradiated in pentane, preferring to react in the conformation shown on the right in order to minimise A1,3 strain. This, along with the electronics of the ring, the length of the tether to the alkene and the tendency of the reaction occur meta (i.e. as a [3 +2] rather than a [2 + 2] or [4 + 2]) meant that the reaction was highly stereo- and regio- selective, forming just two major products out of the God-knows-how-many possible.[6] The desired one was then separated and the cyclopropane was opened reductively under Birch-like dissolving metal conditions. Next, the side chain was then oxidised up to the acid in a couple of steps and the the cyclopentene ring was ozonised.[7] The rather unstable resulting dialdehyde then underwent cyclisation to the lactol, which required just a simple PDC oxidation to complete the target. Lovely.
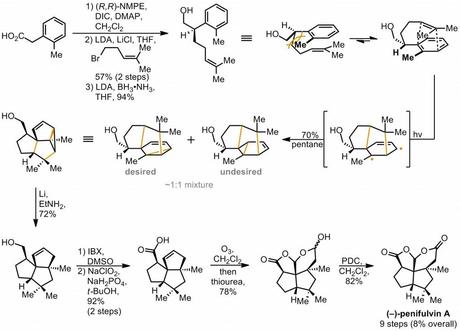
Blue background removed for clarity!
I think this synthesis is definitely one of my favourites from recent years, possibly for the highly creative disconnections alone. It looks pretty simple in the forward direction, but that's part of its charm. As Einstein said, "any idiot can make something complicated...". Tune in next time for more retrosynthetic wizardry in Mulzer's synthesis of echinopines A and B, starting from... cyclooctadiene!
Etc.
- I don’t know the first thing about German but I’ve heard it said a few times in this form. I’m told that it literally means something more like ‘each strategy only works well until you come to the enemy’.
- There’s a recent review in BJOC on the arene-alkene photocycloaddition here (free!). It's probably Reading's greatest contribution to chemistry.
- I later found out that Mulzer's co-author on the penifulvin papers also quite remarkable. Her name is Tanja Gaich (bottom left of slide), and she's just started an academic career at the University of Hanover. During her time in the Mulzer group she managed to finish both penifulvins A and B (one JACS, one Org. Lett. as sole co-author), publish work towards epothilone B and providencin (four papers as first author, two as second) and write four reviews. Then she went to work with Phil Baran on (–)-palau’amine, the (–)-axinellamines and the (–)-massadines. Oh, and remember that much talked about JOC perspective on the ideal synthesis? That was her and Phil. Her first independent publication came out a few weeks back… in Angewandte. One to watch, I think.
- You can find it here [PDF] [SI]. The synthesis of penifulvins B and C is very similar (but still very clever).
- Myers' discusses the use of this reagent in his full JACS paper on the methodology. Reduction of these amides is actually quite tricky, and just using diborane or BH3•THF tends to just give the tertiary amide. Lithium pyrrolidide-borane reagent was found to provide a partial solution to this problem, but didn't work for all substrates. It did, however, inspire the group to create lithium amidotrihydroborate (LiH2NBH3, or LAB) which apparently represents the best solution to this problem. It's easily formed in situ from deprotonation of commercially available BH3•NH3 with n-Buli, or, better, LDA.
- Actually, the [3 + 2] was very regioselective with regard to the reactive positions on the arene ring, but the diradical intermediates can form two regioisomeric cyclopropanes without much preference for one over the other.
- You might be wondering why the acid wasn't just used in the meta-photocycloaddition. As far as I can tell this isn't because it was found to be incompatible with the reaction but was more likely to do with difficulties in hydrolysing the amide after alkylation - the paper complains of extensive racemisation when this was attempted.
