On Monday, See Arr Oh over at Just Like Cooking posted on this non-obvious Diels-Alder reaction recently published by the Vanderwal group, suggesting that it'd make good problem session fodder. And I agree:
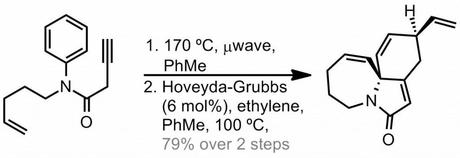
Fortunately, this tied in perfectly with my plans to run our group problem session next week on a pericyclic theme and so it was duly incorporated. If you're interested in what else featured, I also included a question on the origin of the metastability of Dewar Benzene (which I've blogged about before).
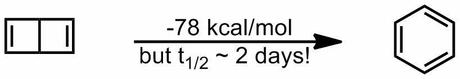
After a few easier questions I finished up by asking people to suggest a mechanism for this interesting sequence published a few years back.
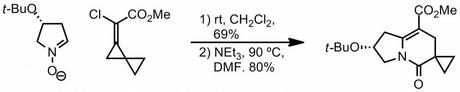
Have a think and then read on for a possible answer!
Okay, here's one plausible mechanism that the authors themselves put forward (J. Org. Chem., 2006, 71, 2417). I find the step that breaks the N-O bond a bit troubling (bottom right), but other than that it's not too bad. The halomethylcyclopropane-halocyclobutane rearrangement is apparently quite a well studied thing.
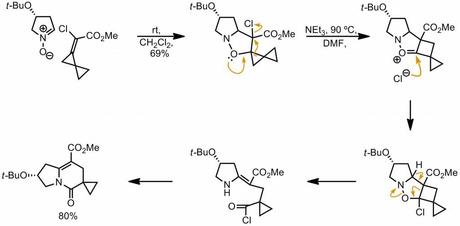
More total synthesis soon, I promise!
