COMT, Catechol-O-methyl transferase, is an enzyme that degrades catecholamines–such as dopamine, epinephrine, and norepinephrine (or adrenaline and noradrenaline as they are called in the UK). It was first discovered in the ’50s by Nobel laureate and pirate Julius Axelrod.

Julius Axelrod
More recently, scientists discovered an evolutionarily recent nonsynonomous single-nucleotide polymorphism (SNP) in the protein-coding portion of the COMT gene–Val158Met or rs4680. This means that some people carry a version of the gene that differs by a single DNA basepair and alters the 158th amino acid in the protein, changing it from valine to methionine.
Just because an amino acid has been altered, doesn’t mean there will necessary be a functional change in the protein, that has to be studied explicitly, but in this case it was dramatic.
Scientists have studied the Val158Met polymorphism and found dramatic effects in its enzymatic activity, expression levels, and behavioral phenotypes for its carriers as well with influences on subjective well-being, emotional processing, and possibly even dopamine-related diseases such as schizophrenia and Parkinsons. It is an extremely well-studied polymorphism with 165 papers referencing it on pubmed.
COMT Val158Met’s biochemical effects
When scientists transfected the Met polymporphism is into cultured cells, protein expression decreased, and it’s overall enzymatic activity greatly decreased. When they controlled for transfection efficiency using pSV-β-galactosidase (though I am not familiar with this technique and how good of a control it is), they saw a 70-90% decrease in immunoreactive protein staining for COMT staining, and a 40% decrease in enzymatic activity measured by the amount of isotopically labled methyl groups were added to 3-4-dihydroxybenzoic acid (Shield et al., 2008). Of course, you always want to study things in as physiological of conditions as possible. Chen et al. (free fultext) did a similar experiment at body temperature in post-mortem human DLPFC tissue and also showed a 40% decrease in COMT enzymatic activity.

So great, the COMT Val/Met polymorphism has robust biochemical effects in the relatively well-characterized and obviously important dopamine pathway, but does it have measurable phenotypic effects in humans?
COMT Val158Met’s human phenotypic effects: card sorting, pot smoking, and happiness
In 2001, Egan et al. showed that the Val/Met polymorphism can effect frontal lobe function. They studied 175 patients with schizophrenia, 219 unaffected siblings, and 55 controls. COMT genotype predicted preservation in the Wisconson Card Sorting (WCS) task (p=.001).
WCS is a neuropsychological test, measuring subject’s “set-shifting,” cognitive flexibility, or executive function ability. The experimenter shows the subject a card, and subjects must choose which pile to place the card into. The only feedback the experimenter gives to subjects is whether they sorted a card correctly or incorrectly, and from this feedback they must learn the sorting rule on their own. At some point during the trial, the experimenter changes the sorting rule , without the subjects being notified. Perseverance errors are made, when the subject continues to sort by the first rule they learned and ignore the new feedback they are getting. Schizophrenics and patients with damage to the dorsal-lateral prefrontal cortex (DLPFC) perform poorly on this task; they often perseverate and learn the second rule more slowly than controls. (I’ve previously written about the DLPFC’s involvement in economic decisions.)
Here’s one of the really interesting parts. The low activity Val/Met polymorphism, which raises dopamine levels, ENHANCED cognitive performance. This may seem counter intuitive if like me you had at one point learned the over-simplified dopamine hypothesis that basically went: dopamine antagonists work as antipsychotics, therefore schizophrenics have too much dopamine. Of course, the brain is never that simple and location is important. According to current consensus, schizophrenics are often hypodopaminergic in the frontal cortex–the most-involved region in executive function tasks like the WCS–and hyperdopaminergic in other regions such as the striatum. Interestingly, giving schizophrenics dopamimetic drugs like cocaine and amphetamines actually improves their performance on this task.
While COMT is expressed in most of the brain, it may have a stronger effect in the prefrontal cortex. This is supported experimentally by the fact that COMT knockout mice (that do not express the gene at all) show increases in dopamine in the prefrontal cortex but not the striatum (Gogos 1998). Theoretically, it is supported by the observation that the PFC has much lower levels of dopamine transporter (which has a 1,000-fold higher affinity for dopamine than does COMT). So while in the striatum, the effect of the dopamine transporter probably dominates, internalizing dopamine where it is broken down by the intracellular enzyme mono amine oxidase (MAO), in the PFC far less dopamine is being internalized and far more is being broken down in synaptic or parasynaptic regions by COMT, which acts extracellularly.
COMT Val158Met Marijuana and Schizophrenia
Caspi’s 2005 longitudinal cohort study of over 953 caucasian NZ children, were followed until they were 26-years-old, and showed that children who carried the COMT val158 allele and used cannabis before age 18 were more likely to exhibit psychotic symptoms or develop schizophreniform disorder. Cannabis use had no influence on psychosis in individuals with two methionine alleles.

Of the cohort, 1% of the group met DSM IV criteria for schizophrenia (symptom duration > 6 months), and another 2.6% for schizophreniform disorder (i.e., symptom duration from 1–6 months). The cohort was tested for broader pyschosis-like measures, and 13% reported >1 non-drug-related hallucinatory experience, and 21% reported >1 delusional belief symptom (8% reported both and 26% reported either). These numbers may seem scarily high, but similar numbers are reported in studies of the North American population as well.
Using the regression:
Psychosis=b0 + b1(COMT) + b2(adolescent – onset cannabis use) + b3(COMT × adolescent-onset cannabis use)
there was a significant, but not super-robust interaction on the additive scale between COMT and cannabis use before age 18 for predicting schizophreniform disorder (χ2(1) = 4.5, p = .034) and evidence of hallucinatory experiences (χ2(1) 4.9, p = .027), but not for evidence of delusional beliefs (χ2(1) = .73, p = .39).
However, these findings were not supported by Zammit’s 2007 study of 493 schizophrenic patients’ COMT genes (among others) and their interactions with cannabis use. The study found no association between Val158Met genotype and cannabis use in our sample of 493 persons with schizophrenia. (And I guess, if the Val allele x marijuana environmental interaction was causative of schizophrenia, you’d expect a larger percentage of the smokers with schizophrenia to have the allele. Although they claim they should have enough statistical power to replicate the interaction, they admit it is not the best design to assess the gene x environment interaction.)
Other studies have claimed that the Val/Met polymorphism is a risk for schizophrenia in and of itself, but while the region does have some signal in genome wide association studies (GWAS) of the disease, the signal is not very strong, so any association is unlikely to be big. Therefore, if there is a strong association, it will likely involve a gene x gene interaction or gene x environment interaction.
COMT has been looked at as a candidate gene in schizophrenia and psychosis, because of the involvement of dopamine in those diseases. Also, COMT it is one of the genes where a copy is lost in the 22q11.2 deletion syndrome, also known as DiGeorge Syndrome or VCS, which increases the relative risk of schizophrenia from around 1%, to around 20-30%.
COMT Val158Met and Pleasure
In my opinion, one of the most intriguing findings about this polymorphism is its apparent effects on the experience of pleasure. Not only does the met polymorphism seems to improve prefrontal cortex function, and possibly decreases psychosis risk, it actually makes people report better well-being.
Using a cool psychological technique called experience sample monitoring (ESM), Wichers et al. 2008 genotyped 492 subjects and gave them digital wristwatches and self-assessment forms. For 5 days, the watches would beep at a random time in ten blocks between 7:30 and 22:30. So for example, the watch might beep at 8:37, then 11:14, and so forth throughout the day, and at each beep the subject was told to record their “thoughts, current context (activity, persons present, and location), appraisals of current situation and mood.”
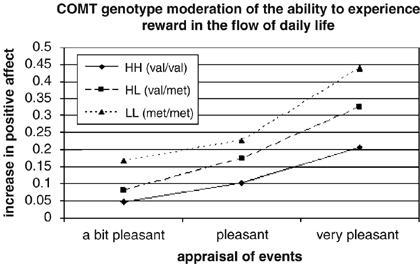
“Reward experience on the continuum of event appraisal stratified by COMT genotype (event reference category is ‘neutral’).” Wichers (2007) Neuropsychopharmacology
Subjects with the met polymorphism reported more pleasure from what should have been equally pleasurable activities, and the effects were got bigger the more pleasurable an event was.
This made me curious about COMT’s possible relationship to depression. However, the first two studies I found in a very haphazard literature review were the opposite of what I expected, with the Met allele being associated to postpartum depression and depression in men (Comasco 2011) and (Åberg 2011). However, perhaps this was to be expected as pleasure and happiness can be very different things, and antidepressants are thought to work through serotonin and norepinephrine pathways, not dopamine pathways (though COMT will effect NE levels).
So what genotype are you?
The evolutionarily recent Met polymorphism isn’t rare by any means. Though allele frequency varies by geographic ancestry–about 12% of the overall population sampled is homozygous for the Met allele, and another 42% carry one copy (see refSNP). If you really must know your genotype, it is measured in the SNP panel done by 23 and me. I think, I’m going to try to hold out for the thousand dollar genome and then get myself sequenced, or see if I can weasel my way into the control group (or experimental group) of some study.
——–
Edit: Since this post has been popular–for my lowly blog anyways ; ) –I wanted to add a bit of background:
Keep in mind that these “candidate gene” studies are notoriously hard to replicate–not that many people actually try to replicate them exactly, as I showed with the conflicting studies with marijuana. Some studies don’t account for multiple comparisons, and unfortunately, some that say they did may be lying in order to get publishable positive results.
Further, even if a relationship is found, it could be that it’s not from the studied SNP but another nearby polymorphism in linkage with it in a given geographic-ancestry population. In other words if the studied SNP and the nearby true causative polymorphism were both found in a common ancestor, you may get a signal from the studied SNP since it is often included in the same chunk of a chromosome during meiosis. For example, in a given background out of people with SNP A at one location on a chromosome, 90% SNP B at nearby location. When you form sperm or eggs, each somatic chromosome is a combination of the chromosome you inherited from your mother and father’s chromosome, but the way they combine with each other genes that were near each other tend to be passed together, so nearby polymorphisms are said to be linked (inversely proportional to the distance between them) and passed down for thousands of generations. And because mutation events are so rare, many of out polymorphisms do go thousands of generations back. However, there is always the small chance that when the chromosomes you inherited from your mother and father combine in gametogenesis (formation of egg or sperm), the recombination event (or splitting and fusion of the two chromosomes), will occur between the nearby genes and because of this linkage is never perfect.
Linkage, complex interactions, and environmental interactions can also make studies hard to generalize and replicate. Linkage can also vary between geographic backgrounds, depending on what SNPs common ancestors in those populations had, so if a study finds a true effect, but through linkage and not the causative SNP, the relationship might not hold up in a different population. Alternatively with complex traits, there may be additive or multiplicative interactions between different polymorphisms leading to the phenotype, which may vary in prevalence in different populations. We’re still really bad at studying these combinatorial relationships, because there are so many possible that they quickly add up and lower the statistical power of your study. Finally, gene x environment interactions are the rule, not the exception, even for highly heritable traits–if someone is starving it doesn’t matter how many “tall genes” they have.
Still, despite all these caveats, as far as candidate genes and SNPs go, the COMT Val/Met polymorphism is a great candidate, because of it’s robust effects, and the gene’s important location in the catecholamine degredation pathway.
If you have any opinions about gene candidate studies, GWAS, reductionist genetics, neurogenetics, etc., or disagree with anything I’ve written here, please leave a comment (especially if it’s snarky.)
Also, if you’ve read this far, you may be intersted in my other posts genetics posts:
Genoeconomics – Can genetic variation predict economic decisions?
New sequencing study links autism to FMRP, the gene disrupted in fragile X syndrome and a regulator of synaptic plasticity
Mirror Touch Synesthesia and the Genetics of Synesthesia
Genetics of Sleep – How much do you need?
Epigenetics and The Brain — 5-hydroxy-methocytosines
——
References:
Shield, A. J., Thomae, B. A., Eckloff, B. W., Wieben, E. D., & Weinshilboum, R. M. (n.d.). Human catechol O-methyltransferase genetic variation: gene resequencing and functional characterization of variant allozymes. Mol Psychiatry, 9(2), 151-160. Nature Publishing Group. Retrieved from http://dx.doi.org/10.1038/sj.mp.4001386
Jingshan Chen, Barbara K. Lipska, Nader Halim, Quang D. Ma, Mitsuyuki Matsumoto, Daniel R. Weinberger, et al. (2004) Functional Analysis of Genetic Variation in Catechol-O-Methyltransferase (COMT): Effects on mRNA, Protein, and Enzyme Activity in Postmortem Human Brain Am J Hum Genet. 2004 November; 75(5): 807–821. Published online 2004 September 27. Correction in: Am J Hum Genet. 2005 June; 76(6): 1089.Egan, M. F., Goldberg, T. E., Kolachana, B. S., Callicott, J. H., Mazzanti, C. M., Straub, R. E., Goldman, D., et al. (2001). Effect of COMT Val108/158 Met genotype on frontal lobe function and risk for schizophrenia. Proceedings of the National Academy of Sciences , 98 (12 ), 6917-6922. doi:10.1073/pnas.111134598
Gogos, J. A., Morgan, M., Luine, V., Santha, M., Ogawa, S., Pfaff, D., & Karayiorgou, M. (1998). Catechol-O-methyltransferase-deficient mice exhibit sexually dimorphic changes in catecholamine levels and behavior. Proceedings of the National Academy of Sciences , 95 (17 ), 9991-9996. doi:10.1073/pnas.95.17.9991
Avshalom Caspi, Terrie E. Moffitt, Mary Cannon, Joseph McClay, Robin Murray, Ian W. Craig, et al. (2005). Moderation of the Effect of Adolescent-Onset Cannabis Use on Adult Psychosis by a Functional Polymorphism in the Catechol-O-Methyltransferase Gene: Longitudinal Evidence of a Gene X Environment Interaction, Biological Psychiatry, 57(10), 0006-3223. doi:10.1016/j.biopsych.2005.01.026.
ZAMMIT, S., SPURLOCK, G., WILLIAMS, H., NORTON, N., WILLIAMS, N., O’DONOVAN, M. C., & OWEN, M. J. (2007). Genotype effects of CHRNA7, CNR1 and COMT in schizophrenia: interactions with tobacco and cannabis use . The British Journal of Psychiatry , 191 (5 ), 402-407. doi:10.1192/bjp.bp.107.036129
Wichers, M., Aguilera, M., Kenis, G., Krabbendam, L., Myin-Germeys, I., Jacobs, N., Peeters, F., et al. (2007). The Catechol-O-Methyl Transferase Val158Met Polymorphism and Experience of Reward in the Flow of Daily Life. Neuropsychopharmacology, 33(13), 3030-3036. American College of Neuropsychopharmacology. Retrieved from http://dx.doi.org/10.1038/sj.npp.1301520
Comasco, E., Sylvén, S. M., Papadopoulos, F. C., Sundström-Poromaa, I., Oreland, L., & Skalkidou, A. (2011). Postpartum depression symptoms: a case–control study on monoaminergic functional polymorphisms and environmental stressors. Psychiatric Genetics, 21(1). Retrieved from http://journals.lww.com/psychgenetics/Fulltext/2011/02000/Postpartum_depression_symptoms___a_case_control.4.aspx
Elin Åberg, Andrés Fandiño-Losada, Louise K. Sjöholm, Yvonne Forsell, Catharina Lavebratt (2011) The functional Val158Met polymorphism in catechol-O-methyltransferase (COMT) is associated with depression and motivation in men from a Swedish population-based study, Journal of Affective Disorders, Volume 129, Issues 1–3, Pages 158-166, ISSN 0165-0327, 10.1016/j.jad.2010.08.009.