 (Credit: Flickr @ Heather Kennedy http://www.flickr.com/photos/moria/)
(Credit: Flickr @ Heather Kennedy http://www.flickr.com/photos/moria/)Researchers at the SLAC National Accelerator Laboratory and Stanford have found that the even glue that binds together the materials in the cathode can have a significant influence on the battery performance. Tweaking that material, which binds lithium sulfide and carbon particles together, created a cathode that lasted five times longer than earlier designs.
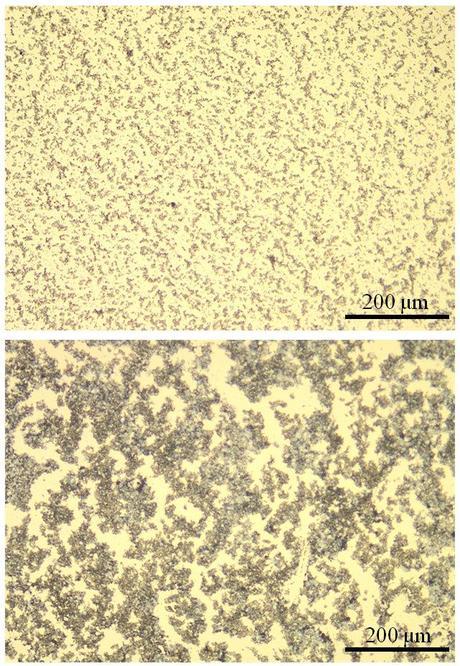
A new binder material forms a fine-grained (top) lithium sulfide/carbon composite cathode, compared with the large clumps (bottom) that form when another common binder is used. In an operating lithium-ion battery, the larger clumps caused the battery to be ruined after just 100 charge/discharge cycles. In contrast, an experimental battery using the new binder lasted nearly five times longer. (Credit: Zhi Wei Seh / Stanford)
“We were very impressed with how important this binder was in improving the lifetime of our experimental battery,” said Yi Cui, an associate professor at SLAC and Stanford who led the research.
Researchers worldwide have been racing to improve lithium-ion batteries, which are one of the most promising technologies for powering increasingly popular devices such as mobile electronics and electric vehicles. In theory, using silicon and sulfur as the active elements in the batteries’ terminals, called the anode and cathode, could allow lithium-ion batteries to store up to five times more energy than today’s best versions. But finding specific forms and formulations of silicon and sulfur that will last for several thousand charge-discharge cycles during real-life use has been difficult.
Cui’s group was exploring how to create a better cathode by using lithium sulfide rather than sulfur. The lithium atoms it contains can provide the ions that shuttle between anode and cathode during the battery’s charge/discharge cycle; this in turn means the battery’s other electrode can be made from a non-lithium material, such as silicon. Unfortunately, lithium sulfide is also electrically insulating, which greatly reduces any battery’s performance. To overcome this, electrically conducting carbon particles can be mixed with the sulfide; a glue-like material—the binder—holds it all together.
Scientists in Cui’s group devised a new binder that is particularly well-suited for use with a lithium sulfide cathode—and that also binds strongly with intermediate polysulfide molecules that dissolve out of the cathode and diminish the battery’s storage capacity and useful lifetime.
The experimental battery using the new binder, known by the initials PVP, retained 94 percent of its original energy-storage capacity after 100 charge/discharge cycles, compared with 72 percent for cells using a conventionally-used binder, known as PVDF. After 500 cycles, the PVP battery still had 69 percent of its initial capacity.
Cui said the improvement was due to PVP’s much stronger affinity for lithium sulfide; together they formed a fine-grained lithium sulfide/carbon composite that made it easier for lithium ions to penetrate and reach all of the active material within the cathode. In contrast, the previous binder, PVDF, caused the composite to grow into large clumps, which hindered the lithium ions’ penetration and ruined the battery within 100 cycles
Even the best batteries lose some energy-storage capacity with each charge/discharge cycle. Researchers aim to reduce such losses as much as possible. Further enhancements to the PVP/lithium sulfide cathode combination will be needed to extend its lifetime to more than 1,000 cycles, but Cui said he finds it encouraging that improving the usually overlooked binder material produced such dramatic benefits.
Original article by Mike Ross
Zhi Wei Seh, Qianfan Zhang, Weiyang Li, Guangyuan Zheng, Hongbin Yaoa, Yi Cui (2013). Stable cycling of lithium sulfide cathodes through strong affinity with a bifunctional binder Chemical Science, 4, 3673-3677 DOI: 10.1039/c3sc51476e
