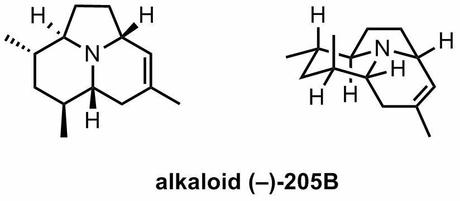
Synthesis of Alkaloid (−)-205B via Stereoselective Reductive Cross-Coupling and Intramolecular [3 + 2] Cycloaddition
Micalizio et al., J. Am. Chem. Soc., 2012, ASAP [PDF][SI][GROUP]
DOI: 10.1021/ja306362m
Here’s a neat alkaloid synthesis published last week by Yang and Micalizio. An earlier JACS methodology paper from the group hinted at an interest in this target, so although this work isn't entirely out of the blue their final route contains a couple of quite unusual steps and is worth a quick look. I must say, the title of the paper is a little long for my taste, but you can't have everything.
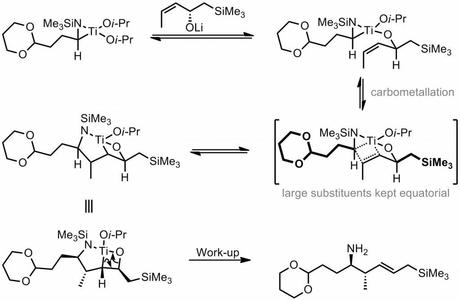
The route begins with the stereoselective synthesis of the homoallylic hydroxylamine required for the planned [3 + 2] cycloaddition. This was achieved via the corresponding amine, which was accessed using a rather non-obvious titanium mediated reductive coupling of an aldehyde and an allylic alcohol. My initial reaction to this step was “huh? Where’s the nitrogen come from?”, and perhaps a bit surprisingly the answer is the LiHMDS itself![1] With the enantioenriched homoallylic amine in hand, the group went on to convert it into the hydroxylamine using a rather uncommon oxidation protocol that involved treatment with benzoyl peroxide followed by hydrazine. At this point you might be asking if a simpler route to this compound, such as asymmetric crotylation of an aldoxime couldn't be used instead. However, it turns out that this alternative approach (and others) was considered (and rejected) by the group and their reasons are laid out in what is probably the longest footnote I have ever seen in a JACS paper.
Anyway, with the hydroxylamine in hand, condensation with butyl glyoxylate led to nitrone formation and the expected [3 + 2] cycloaddition with the tethered alkene occurred in good yield upon heating in toluene. Although this reaction is quite a common sight in modern organic synthesis, the intramolecular version using homoallyic nitrones (as seen here) is a lot harder because these can undergo aza-Cope rearrangements that compete with the desired cycloaddition. Fortunately, in this case, it was found that as long as a glyoxylates were employed, rather than simple aliphatic aldehydes, then the desired reaction did proceed ‘unscathed by potential competitive [3,3]-sigmatropic rearrangements’.
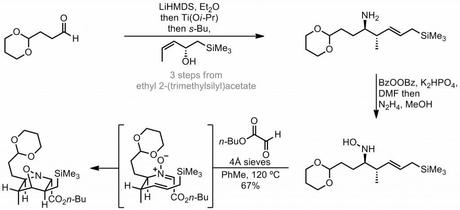
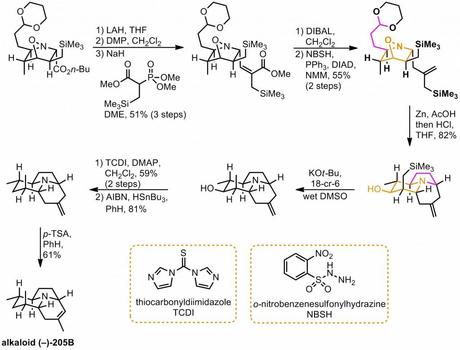
Another advantage of using a glyoxylate-derived nitrone in the [3 + 2] addition step was that it provided a convenient handle to introduce the functionality required for the construction of the remaining ring. Thus, conversion of the butyl ester to the corresponding aldehyde, followed by HWE reaction with the rather unusual phosphonate shown gave the α-trimethylsilylmethylacrylate as an inconsequential 2:1 mixture of diastereomers that were not separated (major shown). The newly installed ester was then reduced to the allylic alcohol and this was then subjected to Myers’ allylic reduction, resulting in transposition of the allylic double bond.[2] Next came the inevitable cleavage of the N-O bond that always seems to follow nitron(at)e cycloadditions, carried out here in good yield using a dissolving zinc reduction. The acid required for this step also had the effect of triggering a reaction cascade involving cleavage of the dioxane protecting group, imine formation (from condensation between the newly unmasked amine and the newly unmasked aldehyde) and, finally, attack on this species by the pendant allyl silane, followed by loss of the TMS group, to form the remaining two rings in style. This well planned and high yielding sequence completed the carbon skeleton of the natural product in a single step and just left a little tidying up – removal of the extraneous TMS and hydroxyl groups and isomerisation of the exocyclic double bond – to complete the target. Thus, the unwanted trimethylsilyl group was removed by treatment with base in wet DMSO (presumably briefly via a primary carbanion) and a Barton-McCombie-type deoxygenation was used to get rid of the spare hydroxyl. Finally, a little bit of acid was used to move the double bond into the ring. A nice route!
Footnotes
- I never seem to do too well at drawing mechanisms on this blog, but I’m quite confident about this one so I’ll give it a go:
2. Although Corey, Schreiber and others had previously converted allylic alcohols to allylic diazenes, and demonstrated that the sigmatropic rearrangement of these intermediates with loss of nitrogen resulted in the overall reductive transposition of the starting alcohol, no convenient protocol for this step was established until Myers’ published this one-pot Mitsunobu based method (Tetrahedron Lett., 1996, 37, 4841). The reaction typically occurs at or below room temperature under quite mild conditions; the only problem is the preparation of the NBSH (which you can think of as nosyl protected hydrazine if you want to make it seem more silly). In fact, the Micalizio paper discussed above contains a footnote that highlights the joy of working with the stuff:
“In our hands, freshly prepared NBSH proved to be quite unstable after drying under high vacuum for 48 h. For example, in the presence of air, a minor tap of the vial containing this material resulted in the generation of red smoke followed immediately by an aggressive flame/ explosion. While the reported preparation of NBSH calls for drying under vacuum at 23 °C for 14 h, in efforts to achieve a similar melting point to that reported in the literature one batch of material was dried under vacuum for 48 h. This was the only modification made to the literature procedure.”
If that’s all a bit exciting, the other way to carry out this transformation is an allylic hydrogenolysis invented by Tsuji which also results in net 1,3-transposition of the double bond. For example, Majetich used Myers’ Mitsunobu trick in his first generation synthesis of perovskone (for the step shown below), but wisely changed to Tsuji’s method for the second generation route:
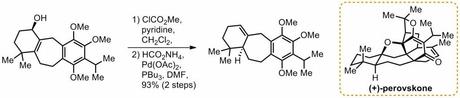 Of course, they both go with inversion - in both cases the new hydrogen atom is added to the opposite side to the starting alcohol.
Of course, they both go with inversion - in both cases the new hydrogen atom is added to the opposite side to the starting alcohol.
