Okay, I've finally got round to a full post on this synthesis. I started writing this almost two weeks ago! More things soon. Enjoy!
Enantioselective Total Synthesis of Hyperforin
J. Am. Chem. Soc., 2013, 135, 644 | [PDF][SI][GROUP]
B. A. Sparling, D. C. Moebius, and Matthew D. Shair,
DOI: 10.1021/ja312150d
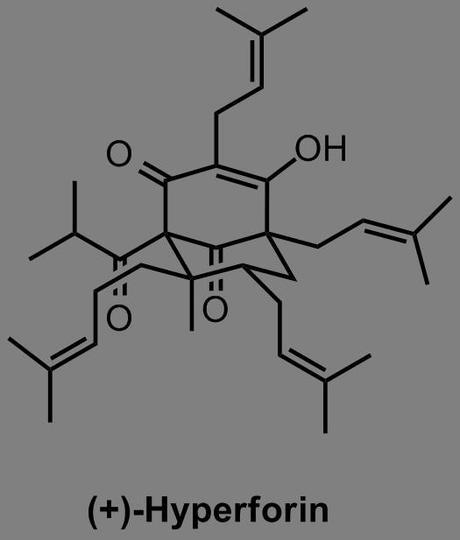

It's a little surprising how few people have made hyperforin, considering that it's been known for over four decades. Indeed, the list of groups with partial solutions and 'studies towards' is an august company indeed: Nicolaou, Chen, Nguyen, Mehta, Jacobsen and several others have all fallen at various hurdles along the way. The compound itself was first isolated in the early seventies from Saint John's Wort (Hypericum perforatum), and is believed to be responsible for the well-documented antidepressent activity of the plant and its extracts, which are popular herbal supplements. No-one's quite sure exactly how it works (although as I understand it that's pretty normal for anti-depressants), and its undesirable physical properties make it an unlikely drug, but perhaps if the right analogue could be made then it could be the next big thing for tormented grad students. Surprising, then, that the only reported synthesis of the molecule before Shair graced the scene was published by Shibasaki in 2010 and although that contained some tasty chemistry (the Fe catalysed asymmetric Diels-Alder and vinylogous Pummerer were my personal highlights), at 51 steps it was perhaps a bit long to do much medicinal chemistry with.[1] The Shair group saw a better way, disconnecting the molecule back to 6 key building blocks:
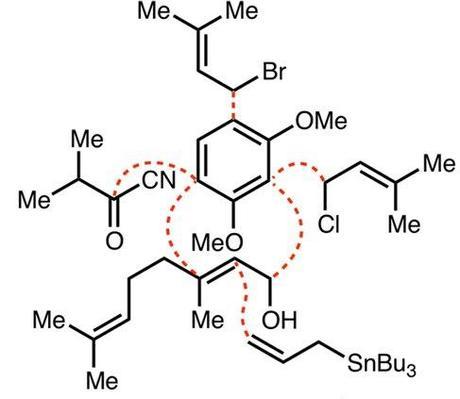
Credit: J. Am. Chem. Soc., 2013, 135, 644
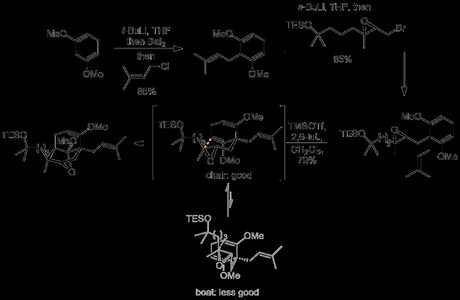
The route began with the Birch reduction of 1,3-dimethoxybenzene to give the bis(enol ether) shown. This was deprotonated with t-butyllithium, the organolithium was then converted to the organobarium (to improve regioselectivity in the alkylation), and this was then reacted with prenyl chloride. Preparation of the organobarium reagent requires a certain finesse, which I wrote about here when the paper first came out, and the SI for this step is worth a look. Anyway, the alkylation product was then deprotonated and alkylated again, this time with the non-racemic bromide shown.[2] Next came one of the highlights of the synthesis: Lewis acid-assisted opening of the sidechain epoxide by one of the nearby enol ethers. This transformation simultaneously set both of the molecule's two quaternary centres: one where the new bond was formed, but also the previously achiral pro-stereogenic centre where alkylation with the bromide had previously taken place (thanks to the epoxide and the chair-like transition state causing reaction with one enol ether to be favoured over the other). And if that wasn't enough, the alcohol formed during the epoxide opening spontaneously underwent ketalisation during the reaction, which saved the group having to protect it for subsequent steps. It's great to see so much achieved in a single step!
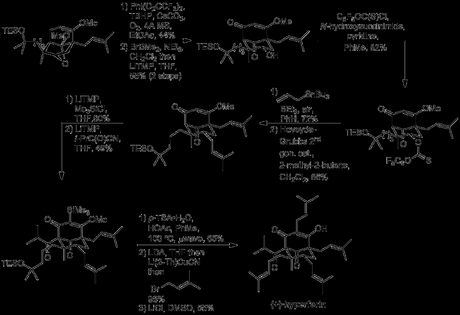
The group now faced the slightly daunting task of oxidising the remaining enol ether to the corresponding vinylogous ester. Allylic oxidations of this type can be very challenging at the best of times, but here things are further complicated by the need for regioselectivity for the the desired position over the 3 other allylic sites present in the molecule. After what I don't doubt was very 'extensive oxidation', the group managed to apply a modified version of Yeung's conditions to obtain a moderate yield of the desired product (and no doubt breathed a sigh of relief).[3] Next, hydrolysis of the ketal was required, to free up the alcohol for further functionalisation. However, this simply transformation was complicated by the unexpected resilience of the ketal (and, presumably, the acid sensitivity of the rest of the molecule). Eventually, a two step procedure was developed where BrBMe2 was used to selectively demethylate the ketal (leaving the TES group unmolested!) to give the hemiacetal which was then treated with LiTMP to eliminate methanol. Conversion of an alcohol to a prenyl group was now required, and in order to achieve this difficult transformation a Keck-type radical allylation was developed. Thus, the alcohol was converted to the corresponding thionocarbonate and treated with allyltributyltin in the presence of BEt3 and air (to initiate the reaction). The stannane effectively served as both allyl source and chain carrier, and the newly introduced allyl group was then extended to the prenyl by cross metathesis with 2-methyl-2-butene.[4] Acylation of the 1,3-dicarbonyl group was up next, but formation of an enolate at this bridgehead position was anything but easy, thanks to Bredt's rule. In fact, so un-acidic was this sp3 methine that deprotonation was not selective over the sp2 methine at the other ketone α-position! The group's solution to this problem took advantage of the fact that although deprotonation was essentially unselective, the reactivity of the sp2 anion was much greater, due its lower steric hinderance. Thus, deprotonation with excess LiTMP, followed by quenching with Me3SiCl resulted in highly selective introduction of a TMS group at this position. Deprotonation with more LiTMP could now be carried out at the bridgehead position, and acylation with isopropyl cyanoformate proceeded in a very respectable yield of 49%.[5] Finally, heating with p-TSA in acetic acid caused simultaneous loss of both silyl groups and elimination of the tertiary alcohol. LDA and prenyl bromide were then used to install the final prenyl group, and demethylation 0f the vinylogous ester by heating with lithium chloride in DMSO gave the natural product. Congratulations to Shair and co-workers for a brilliant synthesis full of clever solutions to difficult problems!
Tangential Information
1. It also had a hideous graphical abstract, and gave the wrong enantiomer, although the latter problem could presumably be fixed fairly easily just by choosing a different ligand for the iron in the asymmetric Diels-Alder. Decide for yourself: Angew. Chem. Int. Ed., 2010, 49, 1103.
2. You probably won't be surprised to learn that this material was prepared in 5 steps from geraniol. Again, the chemistry chosen was solid on scale, and the SI describes the preparation of batches of 172 grams at a time. The source of asymmetry here was the trusty Sharpless epoxidation, and there's probably no other catalytic asymmetric reaction that I'd rather do on scale. Even on 1 mole, the reaction gave a respectable 92% yield and 91% ee!
3. One of the more recent methods for allylic oxidation, this reaction was originally reported in Org. Lett., 2010, 12, 2128. I remember the paper coming out quite clearly because at the time I had just been having problems with an allylic oxidation in a small total synthesis I'd been working on in my spare time. I remember excitedly putting on the reaction, followed by excited TLCing over the following hours, and the eventual realisation that absolutely nothing was happening; I got my starting material back completely unchanged. In fact, until I saw this hyperforin paper I'd never heard of anyone carrying out one of these reactions successfully let alone in such a complicated system, and would have thought it a good candidate for scrutinisation by the good people over at Blog Syn. It's interesting that Shair and co-workers performed the reaction in ethyl acetate, as during a solvent screen by the original authors longer chain esters (i.e. butyl butyrate) were found to be significantly better, but as this reaction is the lowest yielding step in an otherwise great synthesis I imagine that they tried other solvents.
4. This metathesis based allyl to prenyl conversion is a very useful trick if you're into (mero-)terpene synthesis, which I think was first reported by Dirk Trauner. I've used it myself a few times, as it's usually a heck of a lot easier to get an allyl group into a molecule than a prenyl; for example allyl iodide, allylmagnesium chloride/bromide and allyltributyl tin are all commercially available and can be used without concern for regiochemistry (i.e. which end of the prenyl group is electrophilic/nucleophilic). The corresponding prenyl reagents have to be made, and can often react in exciting and unexpected ways. I have made prenyl- (and genanyl-) tributyltin, and apart from a rather unique smell there's little to recommend the experience.
5. Writing this section was made much easier by a detailed description of this sequence left by the 1st author on the paper, Brian Sparling, on my previous hyperforin post in answer to a question by commenter strychnine (emphasis, hyperlinks and comments in square bracket are mind):
"After making compound 18 [lefthand compound, middle line - BRSM], we needed to perform two more C–C bond-forming reactions to essentially complete the total synthesis: bridgehead acylation at C1 and prenylation at C3. Prior work towards hyperforin-like natural products have shown that both positions are similarly reactive (see the deuterium quench discussion in this Danishefsky paper: ACIE 2007 46 8840); however, these positions can be differentiated quite easily given that the C1 position is sterically more congested than the C3 position (see this Simpkins paper for some great examples: JOC 2007 72 4803). Therefore, we first exposed 18 to 5 equiv LiTMP and TMSCl to silylate the C3 position (5 equiv is a bit overkill, but the yields were consistently good at this stoichiometry).
The subsequent bridgehead deprotonation-acylation sequence on S6 took substantially more effort to optimize. Given the steric environment at C1, you’d think that using a smaller amide base would be better for deprotonation, but smaller amide bases contain alpha-protons, and thus these bases (e.g., LiNEt2, LDA) acted as hydride-transfer reagents and reduced the C9 bridging ketone of the substrate. After screening non-amide bases to no avail, we settled on using LiTMP (which contains no alpha-hydrogens). This bulky base only deprotonates the substrate at higher temperature (≤ 0 ºC), but at these temperatures, slow decomposition would take place. And given the fact that the electrophile in this reaction–isobutyryl cyanide–contains an acidic alpha-proton, we found empirically that 3 equiv LiTMP gave the best results for this particular transformation. It was quite a delicate balancing act, but we got it to work reasonably well in the end."
Thanks, Brian - great work!
