As I lay on my couch, shirtless and cold but sweating from my first Covid booster shot, I got a push notification about a Wired article: New Covid Drugs Are Here — and They Could Change the Pandemic
The article highlights the benefits these drugs could bring to the third world, where the logistics of delivering fussy mRNA vaccines that have to be kept frozen has been difficult. But what does these drugs mean, for countries like the US. Where the risk of breakthrough infections, have even the vaccinated feeling hesitant to return fully to normal.
“We’re close to the end of the pandemic.” Those were the words of former FDA Commissioner Scott Gotleib, when he spoke to CBS’s “Face The Nation,” on November 7th, about two new medications that have shown impressive efficacy in human trials. (Note, Gotlieb is on the board of Pfizer, the manufacturer of Plaxovid, one of the two new drugs seeking approval.)



The first drug, the antiviral molnupiravir, has already been authorized in the United Kingdom, and the FDA is scheduled to review the drug on November 30th. The 2nd drug, Paxlovid is a combination of two antivirals ritonavir and PF-07321332. In a randomized, double-blind trial patients treated with Paxlovid showed an 89% reduction in the risk of hospitalization and death at the 28-day mark after beginning treatment.
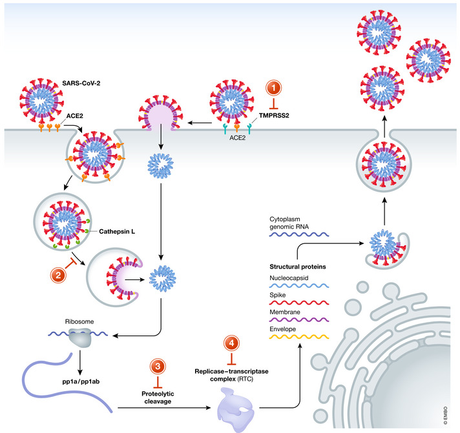
Fig 1. Covid lifecycle. As a cocktail of two protease inhibitors, paxlovid acts on step 3. An ribonucleoside analogue, molnupiravir affects step 4. (figure from Simonis, 2021)
These are small molecule drugs that can be taken orally, which make them easy to manufacture and administer compared to biologics like antibodies or mRNA vaccines. And the fact that paxlovid combines two separate drugs may make it more difficult for coronavirus to develop resistance to these antivirals.
End of a Pandemic?
But is the end of the pandemic phase of coronavirus — and a transition to a present but manageable endemic phase — really in sight?
In the United States, the decline of Delta has leveled off and another winter surge is beginning to brew — right as more move indoors and many travel to see family for Thanksgiving and winter holidays.
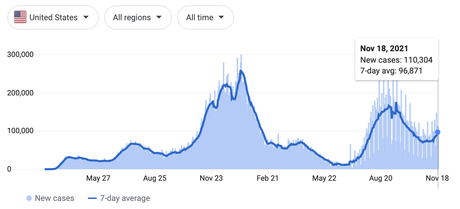
It feels like the only way out of this pandemic is through it at this point. We’re going to need to develop herd immunity one way or the other — whether it be vaccinations or infections.
For large numbers of unvaccinated people, hopefully, these drugs will lessen the deadly impact of those infections. And for the vaccinated who get unlucky, these drugs will offer another level of protection.
A drug just for the unvaccinated?
One strange possibility, though, is that Pfizer’s Paxlovid, might end up only officially approved for the unvaccinated. That’s because the patient population Pfizer studied was not vaccinated (since the unvaccinated are more likely to get severe disease, it’s easier to study the prevention of severe disease in that group). Pfizer has other trials ongoing on lower-risk, vaccinated, patients.
But bioethicist Arthur Caplin told the BMJ, that even if the FDA were to only grant an emergency authorization for unvaccinated people, doctors were unlikely to comply.

