Circuit Neuroscience
Recently, research into psychiatric disease has made great strides, but continued progress may require unpopular and ethically murky research. Joshua Gordon, the new director of the National Institutes of Mental Health writes in this month’s Nature Neuroscience:
“At this unique and exciting time for psychiatry, novel therapies for individuals with mental illnesses seem just around the corner. In particular, recent technological advancements in the study of neural circuits provide reasons to be optimistic that the field is headed in the right direction. Nonetheless, maximizing the chances of translating these advancements into real improvements in patient care requires a carefully considered road map.” – Joshua Gordon, “On Being a Circuit Psychiatrist”
Gordon, a professor at Columbia University, studies ‘circuit neuroscience’—a new term given to the subfield of neuroscience that uses new technologies to monitor and manipulate very specific subgroups of neurons within the brain and measure their behavioral effects.
To give you an idea of circuit neuroscience, here’s a short talk Gordon gave about his research in 2011, describing how the coupling between two brain regions, the hippocampus and medial prefrontal cortex, may be disrupted in anxiety disorders and schizophrenia:
In the time since Gordon gave that talk, his lab has extended these findings from mere correlations to causative mechanisms of disease, in a new study, which shows that, amazingly, inhibiting the connection between the ventral hippocampus and medial prefrontal cortex decreases anxiety in mice.
This kind of finding is really exciting. While my anxiety might be more abstract and existential than a mouse’s (for example, no mouse is worrying about the 2016 presidential election), fear and anxiety have been conserved throughout evolutionary history and their circuitry likely has as well, so there is good reason to think that these findings can be applied to help humans suffering from excess anxiety.
Alright. So we can decrease anxiety in mice, so why don’t we do the exact same thing in humans? Can we specifically inhibit that connection between the ventral hippocampus and medial prefrontal cortex in humans using the same technology we used for mice?
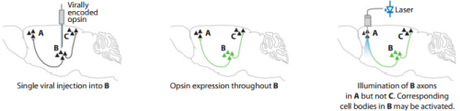
An example of the kind of combinatorial targeting optogenetics allows. From neurowiki.
In the mouse study, Gordon inhibited the specific pathway using optogenetics, a technique that combines molecular genetics to target cells with a certain biological profile, and fiber-optic cables that shine light into a specific part of the brain to combinatorially target and inhibit a group of neurons of interest.
Unfortunately, while theoretically this same approach should work in humans, we can’t implement it using the exact same technology and techniques we’ve developed with mice. For example, In mice, the technology we’ve used to genetically target groups of neurons in mice, only work in mice that have been given mutations that allow those cells to be genetically targeted. (Incidentally a big part of why this type of research is done in mice in the first place.) Additionally, the equivalent circuits and brain areas in humans are much bigger and methods for delivering electric currents, light, or viruses baring optogenetic proteins don’t always scale. Modifying these approaches to work in humans is going to take some troubleshooting in bigger animals. And here is where Gordan in his opinion piece has buried the lead:
“For some questions, like how to transduce and illuminate large brain regions, any large animal model might suffice. Indeed, attempts to use gene transfer models for the treatment of chronic pain have used large animal models to induce widespread central nervous system expression of pharmacogenetic effectors13. But for other questions, such as how to direct gene expression to specific prefrontal cortical regions, only primate models will suffice14. Given the current societal pressures against nonhuman primate research, strong ethical as well as scientific foundations will be required in order to facilitate this important work.” –“On Being a Circuit Psychiatrist”
The Primate Paradox – Ethics of experimenting on Non-human Primates and the ethics of experimenting on humans
Gordon clearly has protecting the need for primates model organism on his mind and with good reason. In late 2011, the United States Institute of Medicine declared that “most current use of chimpanzees for biomedical research is unnecessary,” and the NIH began to dramatically cut funding of research and retire laboratory animals. In 2015, a new rule by the the U.S. Fish and Wildlife Service (FWS), ended a loophole allowing invasive research on chimps despite their endangered species status and effectively banned biomedical research on chimps in the U.S.
Then this September, twenty-one eminent scientists and naturalists, including David Attenburough and Jane Goodall (both of whom’s work I love), signed an open letter they sent to The Independent newspaper calling for the end of neuroscience research on all primates that involves deprivation from fluids and movement restraint, citing a recently published article by Bailey and Taylor in the journal Alternatives to Laboratory Animals. Bailey and Taylor take a more extreme stance that recordings from primates have been made obsolete, and are no longer worth the harm they cause, because of advances in techniques that allow us to record from humans: either non-invasively through techniques like fMRI or through invasive recordings performed alongside neurosurgeries on patients being treated for diseases.
Translational research on primates has a fundamental ethical paradox: the similarity between primates’ brains and our own makes an intervention that was effective on a primate likely to also be effective in humans, but it also means we need to be more careful when we consider whether these experiments are ethical given the pain or suffering they might cause (despite anesthesia and pain medications being given to animals). We must also consider what will happen if we don’t perform this experiments, is it more ethical to perform these same experiments on people—people so afflicted by disease that they are willing to try experimental brain surgery?
One of my uncles suffered from Parkinson’s, a disease that makes movements slow, rigid, and difficult to initiate, and often accompanied by a tremor in the hands and distortion of speech. As a retiree and avid golfer, the tremor and movement problems seemed especially cruel. At first his symptoms could be treated with medication, but after the meds stopped working, as they do in all cases of Parkinson’s, my uncle elected to undergo a radical treatment, one of the first and most circuit neuroscience treatments in medical history.
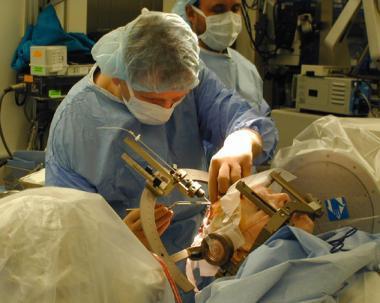
Doctors performed neurosurgery on my uncle, cutting open his skull, and implanting a device, known as a deep brain stimulator, into a precise location of the brain, the subthalamic nucleus, After the surgery, when they turned the stimulator on his symptoms were greatly improved, and he went from a man who was incapacitated to a man who could again play golf.
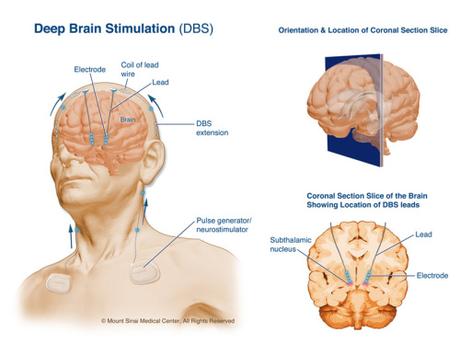
Deep brain stimulation, and all neurosurgery for that matter, has a long history of research on primates, going all the way back to experiments done on primates by Horsely and Clarke in 1908, which developed the devices for accurately targeting regions within in the brain, and paved the way for neurosurgery. STN deep brain stimulation in particular was researched on a Parkinson’s model in macaque monkeys, as Bailey and Taylor acknowledge in their review of the ethics of Nonhuman Primate Research that spurred the open letter:
“Advocates of NHP [Non Human Primates] research claim that macaque experiments (subsequent to the development of the so-called ‘MPTP macaque model’ of PD in 1983) have been indispensable to its development, and specifically to the development of DBS of the subthalamic nucleus (STN). Indeed, this argument, in addition to the others described in this review, is repeatedly used as a ‘flagship’ to showcase NHP neuroscience as a means of convincing the public, as well as regulators and legislators, that NHP neuroscience is vital to progress in neurological medicine (e.g. 17, 41, 88–92). However, in common with BOLD fMRI above, this is of historic interest only, and has little or nothing to add to the critical consideration of the current and future value of NHP neuroscience.”
Bailey and Taylor then further argue that non-human primate research may have been unnecessary for researching Deep Brain Stimulation of the STN. They cite the history experimental work on humans that preceded the creation of the MPTP macaque model of Parkinson’s as evidence that the macaque research was unnecessary. They concede that the deliberate targeting of the STN (as opposed to surrounding regions) emerged from the macaque research, but they think that these discoveries would have eventually been made through human research.
However, I feel they miss some key points both scientific and ethical. For example, they argue post-mortem anatomical studies following electrode implantations can be done in humans and may even give better data than experiments performed on non-human primates. While this is true, human patients like my uncle, often live for years after these surgeries are performed, so researching only humans would slow down the pace of such experiments by orders of magnitude. And with millions of patients suffering around the world, the speed of these discoveries is imperative. Additionally, Bailey and Taylor, insist that the same discoveries would have eventually been made by experimenting on humans (like my uncle), but seem to ignore the ethical problem of imposing unnecessary risks of those experiments on humans.
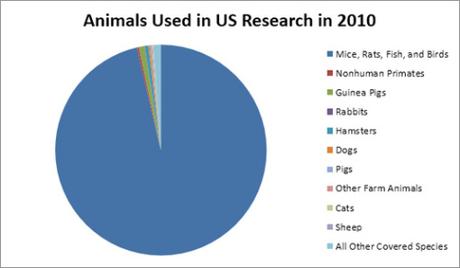
Sources: Annual Report Animal Usage by Fiscal Year, United States Department of Agriculture, Animal and Plant Inspection Service, July 2011. From goo.gl/OSD6KR.
Conclusions
I understand why people oppose research on non-human primates and why we need to consider these ethical questions. Frankly, I’m not sure I could perform the experiments myself. At the same time, I know that many of the people who work with non-human primates are doctors working to cure diseases they have to see patients suffering from every day. I also know many researchers who studied neuroscience or went into research to work on diseases that affected their friends or family. Hearing about my uncle’s experience influenced the lab I joined in graduate school.
At the same time, I also know, not from personal experience but from the history of the field that some callow scientists have disregarded the life of animals in laboratories, and we need systems put in place to prevent those kinds of acts.
Does research that involves deprivation from fluids and movement restraint (that David Attenburough and Jane Goodall signed the letter protesting) fall into that category? I’m not sure.
The laboratory I work in at Tokyo University uses mice. Every year, the labs in our institution gather for a “doubutsu jikken kuyo,” a memorial service to honor the animals sacrificed in scientific research.

Picture of a Doubutsu Jikken Kuyo held at Nippon Pharmaceutical University in 2009.
Two days ago I attended this year’s memorial. I thought about the nonhuman primates who unwittingly, and unwillingly sacrificed their lives. I thought about my Uncle, who died this year and whose memorial service I could not attend, and how he benefited from circuit neuroscience therapy. And, I thought about how this is just the beginning of circuit neuroscience, and how this science will incrementally move forward—but only, if we allow it.
If you oppose research on primates, I ask you to consider the millions of people suffering from psychiatric diseases and neurodegenerative diseases that stand to benefit from potential therapies, and the risks they will have to take in searching for cures, if we don’t perform experiments on primates.
To repeat the quote from the start of this essay:
“At this unique and exciting time for psychiatry, novel therapies for individuals with mental illnesses seem just around the corner. In particular, recent technological advancements in the study of neural circuits provide reasons to be optimistic that the field is headed in the right direction. Nonetheless, maximizing the chances of translating these advancements into real improvements in patient care requires a carefully considered road map.” – Joshua Gordon, “On Being a Circuit Psychiatrist”

