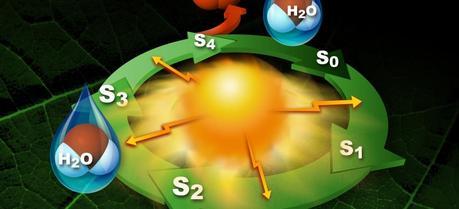
 Photosytem II utilizes a water-splitting manganese-calcium enzyme that when energized by sunlight catalyzes a four photon-step cycle of oxidation states (S0-to-S3). When S3 absorbs a photon, molecular oxygen (O2) is released and S0 is generated. S4 is a transient state on the way to S0. (Credit: SLAC National Accelerator Laboratory)
Photosytem II utilizes a water-splitting manganese-calcium enzyme that when energized by sunlight catalyzes a four photon-step cycle of oxidation states (S0-to-S3). When S3 absorbs a photon, molecular oxygen (O2) is released and S0 is generated. S4 is a transient state on the way to S0. (Credit: SLAC National Accelerator Laboratory)An international collaboration of scientists led by researchers with the Lawrence Berkeley National Laboratory (Berkeley Lab) and the SLAC National Accelerator Laboratory has taken detailed “snapshots” of the four photon-step cycle for water oxidation in photosystem II, a large protein complex in green plants.
The researchers were working at SLAC’s Linac Coherent Light Source (LCLS), the world’s most powerful x-ray laser.
SEE ALSO: Scientists Use Cobalt-Based Catalysts to Replicate Photosynthesis
Photosystem II is the only known biological system able to harness sunlight for the oxidation of water into molecular oxygen.
“An effective method of solar-based water-splitting is essential for artificial photosynthesis to succeed but developing such a method has proven elusive,” says Vittal Yachandra, a chemist with Berkeley Lab’s Physical Biosciences Division and one of the leaders of this study. “Using femtosecond x-ray pulses for the simultaneous collection of both x-ray diffraction (XRD) and x-ray emission spectroscopy (XES) data at room temperature, we have gone around the four-step catalytic cycle of photosynthetic water oxidation in photosystem II. This represents a major advance towards the real time characterization of the formation of the oxygen molecule in photosystem II, and has yielded information that should prove useful for designing artificial solar-energy based devices to split water.”
Photo-oxidation of water by photosystem II is responsible for most of the oxygen in Earth’s atmosphere. At the core of photosystem II is a manganese-calcium (Mn4Ca) metalloenzyme complex that when energized by solar photons catalyzes a four photon-step cycle of oxidation states (S0-to-S3) that ultimately yields molecular oxygen. Scientists need to observe intact x-ray crystallography of the Mn4Ca ion in action but the molecule is highly sensitive to radiation. The LCLS is the world’s only source of x-rays capable of providing femtosecond pulses at the high intensities that allow intact photosystem II crystals to be imaged before they are destroyed by exposure to the x-ray beams.
“In an earlier study at the LCLS, we reported combined XRD and XES data from photosystem II samples in the dark S1 state and the one visible-flash illuminated S2 (1-flash) state,” says Junko Yano, a chemist also with Berkeley Lab’s Physical Biosciences Division and also a leader of this research. “In this new study we report data from the S3 (2-flash) and S0 (3-flash) states, which are the intermediate states directly before and after the evolution of the oxygen molecule. In addition, we report data for the first time from a light-induced transient state between the S3 and S0 states, which opens the window for elucidating the mechanism of oxygen-oxygen bond formation that occurs between these two states.”
XRD data of all the flash states studied revealed an anomalous diffraction signal from Mn that is not complicated by signals from the overall protein matrix of carbon, nitrogen, oxygen and other metals, or even by the Ca atom, which is a part of the five atom Mn4Ca metalloenzyme complex.
“The detection of this anomalous Mn scattering signal not only validates the quality of our data, but also the procedures used for analyzing the data,” says computational scientist Nicholas Sauter. Sauter and Paul Adams, both with Berkeley Lab’s Physical Biosciences Division and both contributors to this study, are leading an effort to develop new and better methods for analyzing data from the LCLS.
Junko Yano (far left) and Vittal Yachandra (far right) are leading a team of Berkeley Lab researchers in an international collaboration to unlock the secrets behind photosystem II’s ability to split water molecules. (Credit: Roy Kaltschmidt / Berkeley Lab)
Yachandra and Yano believe that the detection of an anomalous Mn scattering signal opens up the possibility for detecting changes pertaining only to the Mn cluster as it advances through the S-state cycles and the oxygen-oxygen bond formation, which is where the catalytic action is taking place.
“Knowing how this happens is important for understanding the design principles used in natural photosynthesis,” Yachandra says.
Results of this study have been published in Nature Communications. The paper is titled “Taking snapshots of photosynthetic water oxidation using femtosecond X-ray diffraction and spectroscopy.” Yachandra, Yano and Uwe Bergmann, a physicist with SLAC, are the corresponding authors. The lead author is Jan Kern, a chemist with joint appointments at Berkeley Lab and SLAC. In addition to Berkeley Lab and SLAC, the international collaboration also included scientists from Umeå University in Sweden, Humboldt University in Germany, and the ESRF in France. (See additional information for a link to the paper, and a full list of co-authors and funding support.)
Kern, J., Tran, R., Alonso-Mori, R., Koroidov, S., Echols, N., Hattne, J., Ibrahim, M., Gul, S., Laksmono, H., Sierra, R., Gildea, R., Han, G., Hellmich, J., Lassalle-Kaiser, B., Chatterjee, R., Brewster, A., Stan, C., Glöckner, C., Lampe, A., DiFiore, D., Milathianaki, D., Fry, A., Seibert, M., Koglin, J., Gallo, E., Uhlig, J., Sokaras, D., Weng, T., Zwart, P., Skinner, D., Bogan, M., Messerschmidt, M., Glatzel, P., Williams, G., Boutet, S., Adams, P., Zouni, A., Messinger, J., Sauter, N., Bergmann, U., Yano, J., & Yachandra, V. (2014). Taking snapshots of photosynthetic water oxidation using femtosecond X-ray diffraction and spectroscopy Nature Communications, 5 DOI: 10.1038/ncomms5371
