[Part 3 of 7]
“The Nature of the Chemical Bond. III. The Transition from One Extreme Bond Type to Another.” Journal of the American Chemical Society, March 1932.
In his third paper exploring the nature of the chemical bond, Linus Pauling dug into the unsolved question of how molecules transition from one kind of bond type to another. While it had been determined that molecules do switch from one kind of bond to another – from an ionic bond to an electron-pair bond, for example – the specifics of how that transition happens remained elusive.
Prior to the third paper, two prevailing ideas were being debated by chemists. One concept, as Pauling wrote, was that “all intermediate bond types between the pure iconic bond and the pure electron-pair bond” exist in some kind of infinite transitionary state. A contrary viewpoint put forth instead that molecules “transition from one extreme bond type to another” in an abrupt manner. Pauling suggested that the answer lie somewhere in between.
In order to determine how molecules transition, Pauling first needed to establish the bond structures of given molecules in their initial states. He did so by defining the bonding characteristics of molecules, a task that takes up the majority of the paper. But amidst this discussion, Pauling arrived at several key conclusions.
To begin, Pauling described many cases where a relationship existed between atomic arrangement – as determined by x-ray crystallographic analysis – and bond energies. When, for instance, a strongly electropositive and strongly electronegative molecule bonded, it was reasonable to assume that the bond was ionic. This presumed, Pauling then used electron energy curves to show that an example group, the alkali halide molecules, were strongly ionic, and that they might generally be thought to form ionic bonds.
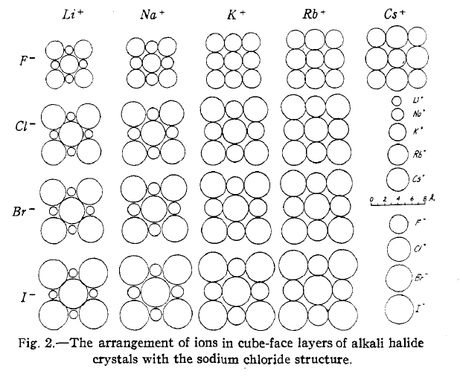
As Pauling pointed out however, these presumptions were faulty. In fact, studies of the bonding in hydrochloric acid (HCl) and hydrobromic acid (HBr) indicated that both molecules were essentially covalent in make-up, whereas hydrofluoric acid (HF) was ionic. So even though it might reasonably have been assumed that the initial states for HCl, HBr and HF would be similar in their bonding, the experimental data indicated otherwise. These findings led Pauling toward the conclusion that there is no single universal answer to the question of how molecules transition, because there is no steadfast rule determining the types of bonds that hold molecules together before they transition.
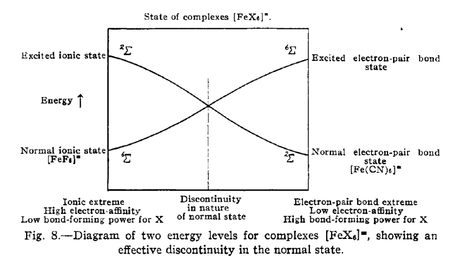
Having arrived at the conclusion that one could not lean upon a guaranteed universal bond type, Pauling then turned to his burgeoning theory of resonance to develop more precise thinking about transition mechanisms. Pauling specifically argued that when bonds transition from one type to another, rather than shifting either abruptly or in a continuous state – as the two competing models then prevailing put forth – they instead shift to an intermediate resonant state before switching to a new bond type.
Pauling was in essence suggesting that, in between classically-defined “completed” bond states, there also existed an intermediate bonding state that could best be understood through the theory of resonance. Moreover, Pauling argued that idealized bonds, such as pure covalent bonds or pure ionic bonds, did not technically exist. Rather, bonds might more accurately be described as constantly transitioning through resonant states, some of which more closely approximated a classic bond type.
Pauling understood that the concept he was putting forth was quite theoretical and that, in practical terms, it was hard to work with molecules if they existed in a constant state of transition. As such, Pauling allowed that, for purposes of discussion, it was acceptable to think of molecules as residing in discrete bonding states. He likewise acknowledged the convenience of using more traditional names (ionic, covalent, etc.) when referring to bonds, even if they never fully existed.
Pauling then concluded that, even though bonds were constantly transitioning, for certain bond types – such as “when the normal states for the two extremes have the same number of unpaired electrons” – it could be assumed that they had transitioned in a continuous state. But, of course, continuous state transition was definitely not always the case and could not be universally applied.
In conducting the work that led to his third paper, Pauling had sought to define a universal rule that would govern the transition between bond types. By the time that he delivered his manuscript though, he had recognized that not only was a universal law unattainable, but that what he did find had its limitations. In particular, Pauling suggested of his approach that “It is not possible at the present time to carry out similar calculations for more complicated molecules,” though “certain less specific conclusions can, however, be drawn.”
Regardless, Pauling’s third paper broke new ground on a topic of keen importance to structural chemists. By applying the theory of resonance, Pauling helped chemists to understand that there was a spectrum of polarity, and that bonds were not always strictly of one kind or the other. Importantly, in this same paper Pauling did not fall prey to dogmatism, and allowed that bonds residing near the ends of one spectrum or another might fairly be said to represent so-called “classic” bond types.
