[Guest post written by John Leavitt, Ph.D., Nerac, Inc., Tolland, CT.]

Linus Pauling lecturing on sickle cell anemia, Kyoto, Japan. 1955
In September 2010, the company BlueBird Bio announced that it had cured a patient with the hemoglobinopathy, beta-thalessemia, by correcting the genetic defect in beta-globin that this patient inherited from his parents. This came 61 years after Linus Pauling and his associate, Harvey Itano, explained the cause of hemoglobinopathies such as sickle cell anemia. Beta-thalessemia like sickle cell anemia is caused by an inherited mutation in the beta-globin gene, just a different mutation. In the case of thalessemia, the defective beta-globin gene product disappears, whereas the defective beta-globin in sickle cell anemia remains stable to wreak havoc on the body. BlueBird Bio accomplished this first cure of a hemoglobinopathy by removing the blood-forming hematopoietic stem cells from the patient, engineering his cells ex vivo with a correct beta-globin gene, and then putting the cells back into the patient. The stem cell transplant sustained itself and produced red blood cells which functioned normally in the circulatory system. For the first time in this patient’s 18 year-old life, he did not have to have a monthly blood transfusion.
In late September 1981, when I gave a seminar at the Pauling Institute of Science and Medicine in Palo Alto, CA, I noticed that Dr. Pauling was smiling during the talk. He was aware of the discovery of the muscle isoform of actin by his friend Albert Szent-Györgyi and knew about the structure and function of actins (the subject of my talk). After reading Dr. Pauling’s 1949 paper on the molecular nature of the sickle cell trait, I understood that he was seeing during my talk the very same experiments in my discovery of a mutant human beta-actin that he and Harvey Itano had performed, which led to the prediction of a mutation in the hemoglobin protein that caused sickle cell anemia. His paper was the very first to describe the molecular genetic basis of a human disease. By 1981 there was plenty of conceptual evidence to suggest how I could look for mutations in proteins using electrophoretic separation of complex mixtures of cellular proteins. In 1949 though, Dr. Pauling was way ahead of his time. In his and Itano’s case, the plan was well thought out based upon years of characterization of oxygen bonding to the heme of the globin molecule. By contrast, I was very lucky to find a mutation in the most abundant structural protein of the cell, cytoskeletal actin in a human fibrosarcoma cell.

Harvey Itano.
It was probably evident in 1949 that hemoglobin amounts to about 95 percent of the total protein of a mature red blood cell; so these cells were essentially bags of hemoglobin molecules – globin polypeptides with attached heme moieties with an iron atom that bound oxygen. The heme-bound iron carries oxygen through the arterial system to cells for respiration. After delivery of oxygen to tissues, these red blood cells (RBCs) return carbon dioxide to the lungs through the venous system for expiration. In sickle cell anemia, after RBCs deliver oxygen throughout the body, the RBCs take on a sickled shape, clog the venous system and lyse, causing a wide variety of systemic problems. Pauling and Itano predicted that this change in RBC architecture was a direct consequence of “two to four” charged amino acid changes in the globin complex, which consists of two beta-globin subunits and two alpha-globin subunits (this was not known then). Because of the science that came after their discovery, we know now that the genetic mutation in the beta-globin moiety is a single amino acid exchange of glutamic acid to valine resulting from a single nucleotide transition (A to T transition) in codon 6 of the beta-globin gene encoding the 147 amino acid polypeptide. Thus two positive charges were added to the hemoglobin molecule by this mutation. Pauling and Itano concluded that these charge alterations caused RBC sickling.
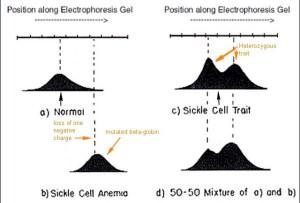
Important discoveries can be quite simple. The figure below is the key experiment carried out by Pauling and Itano, an electrophoretic separation of hemoglobin based upon its isoelectric point (net charge). Because of the mutation in codon 6 present in both inherited beta-globin alleles, the hemoglobin complex migrated to the right of the normal hemoglobin by approximately “two to four” positive charges (panel B compared with panel A). At pH 6.9 the normal hemoglobin was shown to have an isoelectric point of 6.87, migrating as a negative ion, whereas the mutated hemoglobin had an isoelectric point of 7.09 migrating as a positive ion. We know now that this electrophoretic change in the hemoglobin complex described by Pauling and Itano is due to the loss of a single negative charge in a glutamic acid residue (replaced with an uncharged valine residue) near the N-terminus of the two beta-globin moieties of the hemoglobin molecule. Today, the fact remains that this is the only mutation in hemoglobin that causes sickle cell anemia, although other beta-globin mutations cause other hemoglobinopathies like beta-thalessemia. Panel C shows the electrophoretic behavior of hemoglobins in a heterozygous carrier of the disease-causing mutation (Panel D is a control mixture of the globins in panels A and B). Much more insight about these phenomena is discussed in the Pauling and Itano paper but the charge alteration in hemoglobins is the basic observation.
Fast-forward to 1976. I decided to look for evidence of charge-altering mutations in a protein profile of about 1,000 visible proteins (polypeptides) comparing normal and neoplastic cells by looking for Pauling and Itano’s evidence of mutations, e.g. minor charge alterations in proteins in the protein profile. A technique had just been developed by Patrick O’Farrell which permitted high-resolution separation of virtually all major protein gene products of the cell.
An elegant study was performed by Greg Milman at the University of California at Berkeley who demonstrated that one could predict the occurrence of mutations in the relatively minor protein, the enzyme hypoxanthine phosphoribosyltransferase (HPRT), in HeLa cells by the positional changes in the HPRT polypeptide in high-resolution two-dimensional polyacrylamide gels within complex profile of proteins separated both by their charge (isoelectric point) and their molecular weight. When I saw Milman’s result I decided to use this technique to compare normal and neoplastic human cells to see if I could identify charge alterations similar to those demonstrated by Pauling and Itano in hemoglobin and by Milman in HPRT.
I labeled the proteins of normal and tumor-forming human fibroblasts with S35-methionine and separated them using O’Farrell’s two-dimensional technique (isoelectric point separation is a tube gel followed by molecular weight sieving in an SDS slab gel). Then I fixed the proteins in the two-dimensional slab gel and stained these proteins with Coomassie blue dye.
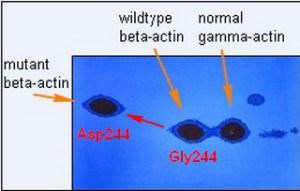
With the dye you could only see the most abundant proteins and I was surprised to see this pattern of actins in the tumor-forming fibroblasts shown above. This image is actually the image of the radioactive protein pattern in the region of actins (pI 5.3 to pI 5.1, molecular weight Mr about 42,000) developed after a very short autoradiographic exposure to X-ray film (a digital image). Normally you only see one beta-actin spot barely separated from the gamma-actin spot. Gamma actin is a second isoform of actin encoded by a separate gene which differs by only four amino acids from beta-actin. Normally there is about twice as much beta-actin at isoelectric point 5.2 as gamma actin and both actins together amount to 5-10 percent of the total cellular protein. But half of the normal beta-actin was missing and a new more negative spot at isoelectric point 5.3 appeared. I was able to show that this was a new form of beta-actin by tryptic peptide separation and other criteria. The observation that the new variant migrated slower in the second dimension as a larger protein was later attributed to a frictional effect in the gel sieve due to an altered conformation caused by the amino acid change.
These observations and other differences in protein expression between the normal and tumor-forming fibroblasts were published in the Journal of Biological Chemistry in February 1980. A second paper was published a month later demonstrating that a T-cell leukemia also had a beta-actin anomaly which suggested loss of a beta-actin allele. It is now well established that reorganization of the actin cytoskeleton occurs when cells become cancerous, although mutations in the structural gene may be less common. These alterations can also be caused by changes in actin-binding proteins.
Later in the year, with my colleagues at the Max-Planck in Goettingen Germany, Klaus Weber and Joel Vandekerckhove, I published the sequences of the normal human beta- and gamma-actins and the mutant beta-actin in Cell. The normal and mutated sequence of human beta-actin is shown in the figure below.
The simple electrophoretic difference between the mutant and normal beta-actin was a single amino acid exchange of a neutral glycine for a negatively charged aspartic acid at amino acid residue 244 in the 374 amino acid polypeptide chain, an observation similar to Pauling and Itano’s hypothesis 32 years earlier. An amino acid exchange at this residue in the actin polypeptide chain had never been observed in any eukaryote. Two years later I cloned the mutant and wildtype human beta-actin genes at the Pauling Institute and formally proved the existence of the mutation at the level of the gene. This mutation was caused by a single nucleotide change in the gene. Several years later my colleagues and I demonstrated that acquisition of this simple mutation contributed to the tumorigenic phenotype of the cells in which it arose.

The sequence of human beta-actin and its amino acid 244 mutation (the most highly conserved protein in eukaryotes).
Ed Note: This week marks the sixth anniversary of the creation of the Pauling Blog. Birthed to help promote the unveiling of a postage stamp, the blog, 461 posts later, has developed into a resource of consequence with an audience that is steadily growing. For those who might be interested in how the project operates, please see this post that we ran one year ago.
As always, we thank you for your continued readership. We plan to keep researching and writing, so please keep coming back!

