By Alycia Halladay, PhD, Chief Science Officer
and the Scientific Advisory Board of the Autism Science Foundation
2015 was an unprecedented year for autism research with many significant advances that will improve the lives of people with autism. Just last week, for example, the CDC reported a 5-month decrease in the age that children are first being evaluated for autism, which means families are learning the signs, acting earlier, and, hopefully, starting early intervention services earlier. In 2015, we changed the way we think about females with autism, gained a better understanding of the underlying genetic causes of autism, and made important progress in both behavioral and medical interventions. Here’s a summary of some of the year’s most significant findings.
Researchers are expanding their attention beyond boys with autism, to the girls and women.
More males are diagnosed with autism than females. This means fewer girls and women participate in research, some scientific conclusions only apply to males, and, in some cases, females are not even studied because of the assumption it is a “male” disorder. This has disadvantaged understanding of females with autism. Luckily, 2015 was a year that science challenged the assumptions that autism is a male disorder.
The journals and news outlets are catching on to the special need to look at females with autism. A special issue of Molecular Autism was published that included many groundbreaking studies on females with autism, including studies involved in early behavior, genetics, and brain structure. A special issue in the journal Autism invited submissions this year, to be published in 2016 or 2017. Also, Spectrum News compiled a summary of what is known about females with autism, from basic science to specific challenges seen in girls and women with ASD. It includes science reports and essays written by women on the spectrum.
So, what did scientific research reveal in 2015? First, the brains of girls with autism are different than boys with autism, specifically regions relating to language function and the way that different regions of the brain connect to each other1,2. Differences in the size of the corpus callosum are seen even before a diagnosis3, and researchers believe they may help explain why symptoms are different in boys and girls.
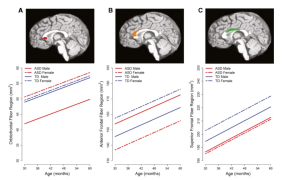
Differences in size of different regions of the brain in boys and girls with and without autism.
The corpus callosum consists of neural fibers that connect the left and right sides of the brain. Very early signs of autism may also be different in boys and girls4, but they are subtle and young boys and girls with autism may be more similar than different.5,6 Findings from the study of the molecular signatures in the brains of males and females with autism are hard to interpret because of the number of female brains with autism to study.7 The sex bias in autism diagnosis is also reflected in a scarcity of biological samples, including brain tissue, from females with ASD. If you are a woman with autism, or the parent of a girl with autism, please consider registering for the Autism BrainNet. It’s easy, and registrants receive regular updates on why this resource is so important.
But it’s not only the girls with autism bringing attention to research in sex differences, it’s the whole family. For example, families in which there is more than one person diagnosed or a female diagnosed tend to show higher levels of autism symptoms both in the affected person and in siblings8,9 and girls with autism show more genetic mutations10. These findings together suggest a protective factor in females which protects against the same symptom presentation as boys.

Undiagnosed sisters play an important role in understanding autism.
Therefore, researchers have started expanding the scope of research from just the individual affected with autism to all family members, so that susceptibility and protective mechanisms from certain symptoms may be uncovered.10,11Earlier this year, the Autism Science Foundation launched a new initiative based on the study of unaffected females called the Autism Sisters Project. You can learn more at www.autismsciencefoundation.org.
It’s not just about an autism diagnosis


One of the questions that has plagued clinicians who make an autism diagnosis is the degree to which the symptoms may change over time, both in the short term and the long term. Two separate studies followed infants with a high probability of a later diagnosis and showed that if a diagnosis was given to a toddler at 18 months, that diagnosis stuck later on in life.12,13 However, that doesn’t mean that the threshold for a diagnosis is always met this early, as a proportion of children were diagnosed at 3 years but not earlier.12,13 Those that received a diagnosis later still showed some of the core features of ASD, but tended to have higher language ability and lower severity of autism symptoms before age 3. There were also distinct patterns across time in each of the groups, reinforcing the variability in presentation of symptoms as well as providing data on how symptoms develop over time in each subset. These findings have enormous clinical importance, as primary care doctors need to continue to monitor children who show early signs and symptoms but do not meet all the criteria of a diagnosis.13,14

Peter Szatmari, MD, led a study following children with autism from ages 2 through 6.
However, while a diagnosis is stable, this does not necessarily mean that delays or impairments stay the same forever. Canadian researchers followed children with autism from age 2 to age 6 and tracked their cognitive ability and their ability to function in daily life (called adaptive behavior). They found that how children started didn’t necessarily predict how they fared later on, as most children with intellectual disability at age 2 didn’t have the same cognitive impairments at age 6.15 While adaptive behavior was more stable, 20% showed improvements in the years after the initial diagnosis.15 These findings show that autism changes and sometimes improves. Further these results underscore that in addition to monitoring autism symptomatology, there is a need to address how children with autism function overall.
Due to lack of funding, researchers studying infants who have a high likelihood of an autism diagnosis tend to stop their studies when the participants are 3 years old. However, a few projects were able to follow families through school age, and the findings may likely alter the way people think about autism. For example, in one long-term study at University of California, Davis, children with and without an autism diagnosis by age 3 were evaluated until they turned 11. The researchers looked beyond ASD and found a higher rate of ADHD symptoms, especially in the girls.16 ADHD and autism often occur together, so while they are different disorders, they may be two diverging roads from the same highway.
Better understanding of underlying genetic causes, and new information on epigenetic mechanisms
In 2015, rapid progress in the genetics of ASD continued. As has been the case in recent years, the lion’s share of progress in identifying specific genes has come through the study of so called germ-line de novo mutations. These are newly occurring changes in DNA that are only in the parent’s sperm or egg cell, are passed on and present in every cell in the child’s body, but are not detectable in the parent’s blood. A new study this year of germ-line de novo mutation bringing together new and previously published data from over 5500 families identified a total 65 genes with strong evidence for a role in ASD risk.10 This study replicated findings girls carry a greater genetic burden than boys, confirming prior findings17 that de novo mutations play an important role even in children with high IQ, and once again highlighted the importance of synaptic function and chromatin modification (discussed later) for the biology of ASD that was reported last year.18 These investigators also found, interestingly, that large copy number variations (CNVs), such as 16p11.2, are very likely to carry many risk genes each with modest risk, rather than one single “smoking gun”.
These findings contribute to the global picture of the genetic underpinnings of autism, which have become much more clear in the past few years. Several papers have shown that variations that are common in the population play an important role in ASD,19,20 in fact carrying most of the risk. This is called “common variation”. Common variation accounts for most of the genetic risk for autism in the entire human population, however, the vast majority of individuals do not have symptoms. It is thought that most people with autism have multiple common variations which increase risk for autism. On the other hand, rare de novo mutations that can affect just one nucleotide of a key autism gene can also play major contributory role.21 In addition to germ line de novo mutations and common risk alleles which are heritable, a recent study from Boston22 highlighted the contribution to ASD of an additional type of mutation, called somatic mosaicism. These are new mutations that occur in the affected individual but sometime after fertilization of the egg. This is most commonly seen in cancer. In order to see the mutation, scientists need to study the organ of interest, in this case, the brain. This was the first time somatic mutations were identified in brain tissue of individuals with
![germlinesomatic[1]](https://autismsciencefoundation.files.wordpress.com/2015/12/germlinesomatic1.gif?w=242&h=183)
Difference between somatic and germline mutations
22But the genetic influence of ASD is not just limited to the sequence of the A’s and C’s and G’s in the code of the DNA or the structure of chromosomes. A growing body of literature is demonstrating that the epigenetic code, or the code that influences how genes are turned on and off, may be just as important. As environmental factors target epigenetic markers in the DNA, this is part of the missing link in understanding gene/environment interactions in ASD.
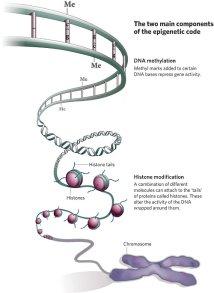
As important as DNA sequence, epigenetic markers affect ASD risk.
One of the reasons geneticists don’t understand epigenetics is that there was no “Human Epigenome Project” to mirror the 2003 Human Genome Project. This is surprising considering doctors are sure that the epigenome contributes to different disorders and diseases, such as diabetes, cancer, and syndromes with a high prevalence of autism like Angelman’s Syndrome and Prader-Willi.23 This year was important for epigenetics research, because a group of researchers published a map of 111 epigenetic markers on the genome from over 183 types of cells, all data has been made publically available for other researchers to study.24 This advance will definitely increase the pace and productivity of science around the role of epigenetics and autism.
In 2012, a gene called CHD8 was reported to increase autism risk.25,26 CHD8 affects chromatin remodeling, or how the DNA is kept tightly wound, which in turn has a major effect on when and where genes are expressed (turned on and off). If CHD8 is disrupted, then DNA does not express itself properly. This year, geneticists looked at what this gene did, and found that it regulated the expression of many other known autism risk genes during fetal development.27. This information makes CHD8 a more interesting gene to understand the bigger picture of brain development in autism. Scientists agree that in the next phase of genetic research, discovery of new genes is going to be matched with figuring out what they do, and how they work together.
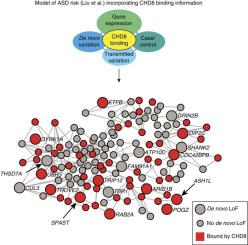
CHD8, involved in chromatin remodeling, is a genetic point of convergence for ASD risk
Another mechanism involved in epigenetics is methylation. This is the attachment of methyl groups to different parts of the DNA sequence that turns off gene expression. In a high-risk siblings study, fathers of a child with ASD showed methylation patterns in sperm that were similar to that seen in the cerebellum of people with autism.28 This suggests that this altered methylation may contribute to autism risk.
Other evidence of gene/environment interactions comes from studies on copy number variations, which are the duplication of or the deletion of parts of a chromosome that can result in the disruption of a gene or genes. Copy number variations contribute to ASD risk, but why they are more often seen in people with autism is still understudied. By looking at environmental factors, researchers have discovered that maternal infection, a risk factor for autism, interacts with the occurrence of these copy number variations to increase ASD symptoms. 29 This finding is a direct demonstration of how environmental factors can increase risk through genetic mechanisms.
Single genes, multiple solutions.
In addition to the more recent discoveries of de novo mutations identified in “typical” autism cases described above, there is a long history of studies of so-called syndromic forms of autism. As more data is accumulated, the distinctions between these groups is becoming less clear, but in general, those genes associated with ASD as well as other characteristic physical features have been considered syndromic, including the Fragile X protein causing Fragile X, or Shank gene causing Phelan-McDermid Syndrome. These are also sometimes described as “single gene” causes of ASD because it appears that a damaging mutation in one of these is sufficient on its own to lead to ASD. As research identifies more of the rare mutations discussed earlier21 the number of these syndromic forms will increase. In the big picture, syndromic/single gene forms of autism account for a relatively small proportion of all ASD cases, however, what they tell us about these syndromic forms can lead to discoveries that impact all forms of autism.

People with Phelan-McDermid Syndrome are often also diagnosed with autism.
In fact, a recent analysis of the shank gene, known to cause Phelan- McDermid Syndrome, shows that mutations of different types of the shank gene are seen across the spectrum of individuals with autism: both those with high IQ and lower IQ and everything in between.30 In other words, this gene doesn’t just show up in those with a certain phenotype, and in fact, one type of mutation shows up in about 2% of individuals with ASD.30 So therapies that were developed for Phelan- McDermid Syndrome may be helpful in treating different types of autism.
Understanding the role of these genes in pathology also helps in understanding the course and symptoms of the disorder. For example, using brain tissue of people with autism, with or without a specific mutation in chromosome 15, neuropathologists showed that there were fewer and smaller neurons in the auditory brainstem–the part of the brain crucial for hearing and distinguishing sounds.31 These cellular deficits are more severe in those with mutation, but the fact that they are present in both groups suggests that there is a common mechanism between auditory problems, pointing to common solutions. In other areas of the brain, scientists see specific persistent and profound decreases in cell size in areas of the amygdala and hippocampus, both involved in emotion, in those with the mutation. 32 These neurobiological differences contribute the phenotype of people with this genetic marker, like lower IQ and high rates of epilepsy. These findings were made possible through postmortem brain tissue donated to the Autism BrainNet.

To register with the Autism BrainNet, go to http://www.takesbrains.org
Postmortem brain tissue is also leading to new targets of treatment. Last year a study reinforced the importance of a type of immune cell in the brain, called microglia, in autism neurobiology.33 This year, a study found that a regulator of microglia activity, the mGLUr5 receptor, less active in brain tissue of people with autism.34 This receptor is also known to be a key target in Fragile X syndrome, and together, this data advances the idea that microglia may help control the shape the shape and size of neurons. When microglia are overactive, this process goes awry and leads to disorders of synaptic plasticity, like autism. This discovery has already led to more research on the role of the microglia in cellular processes with hopes that it will be a feasible treatment target.
New genes that significantly contribute to autism are being discovered. Families are using the power of social media like Facebook to
find each other, and what was once an interesting finding on a gene array is turning into a syndrome. For example, earlier this year m

The big question is whether genetic forms of autism have a hope of being treated. A few years ago, a completely revolutionary study showed that replacing the deficient gene involved in Rett Syndrome reversed the symptoms in a mouse model. 36 This year, the disorder that is produced by too much of this gene, MeCP2, was reversed using targeted gene technologies. 37 Similar modifications of activity of UBE3A, in the area of chromosome 15 associated with autism, have been shown in animal models.38 While it is way too early for clinical trials in humans, the reversal of the behavioral and cellular phenotype in individuals with Rett Syndrome and Dup15 opens the door to targeted gene interventions being available for multiple causes of ASD, even long after the behavioral features present.
New discoveries in treatment: it’s about what you should be doing, and what you shouldn’t be doing.
Many years ago, the first randomized clinical trials on early interventions were conducted. The results of those projects on some early markers of behavior at or around age of diagnosis were published. They were mostly positive, but none of these interventions were shown to improve the core symptoms of autism. Earlier this year, researchers at the University of Washington reported that by 6 years of age, children who had participated in an intervention called the Early Start Denver Model (ESDM) not only maintained the gains attained through this intervention, but continued to improvements in the core symptoms of autism39. So in terms of what families should be doing, early interventions continue to be filed in the “do” category.
There were some interesting findings relating to pharmacological interventions for autism. The hormone oxytocin, otherwise known as the “love hormone,” had previously been shown to benefit individuals with ASD when administered in clinical settings via an intravenous (IV) drip. An IV drip is not really feasible in most real world settings, so researchers studied whether or not a nasal spray of oxytocin would work as well. As it turns out, it did. There were some improvements in some of the social behaviors of autism, but the effects were mild.40 Even so, this therapy is worth following up in additional studies where other interventions are controlled for as well.
Just as important to finding things that help is scientific data on things that don’t. For example, many parents of children with autism turn to dietary interventions like the gluten free casein free (GFCF) diet. Some children with (and without) autism have gluten sensitivities and in these cases, the diet is a good idea. But other parents just don’t know where else to turn and this diet has received enough anecdotal acclaim that they are willing to give it a try. But in many cases there is a large placebo effect, meaning, parents expect or want an effect so much that they unknowingly exaggerate improvement. Very few rigorous research studies have investigated the effects on autism behavior controlling for this placebo effect, until this year. Using a study design that allowed for children to eat either gluten and casein containing or free foods without either them or their caregivers knowing what was in them, the clinicians didn’t see any improvement in autism behaviors41. This casts doubt on the utility of the GFCF diet for treatment of autism symptoms, although, if monitored by a dietician, the diet does not seem to be harmful.
The world of behavioral interventions was turned on its ear earlier this year with a study challenging one of the most common procedures of applied behavioral analysis: repetition. In repetition, a stimulus is presented over and over again to facilitate learning. However, researchers studying repetition in autism found that too much repetition may actually impair the ability of people with high functioning autism to be flexible.42 The implications are clear: when it comes to repetition in some people with autism: maximize with moderation.
Bridging the gap from people in the clinic to people in the community.
Understanding of the early signs and symptoms of autism in the clinic has significantly advanced practice in the pediatrician’s office.43 This year, the same group of researchers that provided the data to enforce early screening for all children with autism challenged primary care providers to look even more closely and take even more action for those with a suspected diagnosis.44 In addition, in the face of the U.S. Preventive Services Task Force (USPSTF) ambiguity on the importance of universal screening for autism, this group continued to urge autism-specific tools to identify autism at 18 and 24 months.44 Finally, they urged culturally- appropriate interventions that involve all family members.

Amanda Gulsrud, PhD, from UCLA, works with a child with autism
As mentioned earlier, a number of rigorous studies examining the effectiveness of early interventions have reinforced the benefits of interventions targeting social, emotional, behavioral domains for the potential prevention of ASD symptoms. While improvements in overall behavior in children and parenting are seen, the effect on specific autism symptoms is unclear.45 On the other hand, reducing behavioral problems in those with autism is a huge concern of parents, and the largest randomized behavioral intervention study on behavioral issues in autism to date compared parent-training to parent education was published in 2015. There were improvements in these behavioral problems in both groups, but parent training was clearly superior. After 6 months of treatment, children in the parent-training group showed a 48 percent improvement on parent ratings of disruptive behavior compared to a 32 percent decline for parent education, and 70% of those in the parent-training group saw an overall positive response by a clinician. 46 The data is encouraging, but data on standardized, objective measures of outcome showed modest improvements in behavior. This study reinforces the value of parent-training models to improve outcomes, and other research in the past year has focused on the right ways to train parents. This means making sure parents are getting the support and feedback they need and delivering the interventions the way they were designed. 47 This is clearly important, as the ultimate goal of developing a behavioral intervention is to make sure that it can be shared with children in a variety of settings under a variety of circumstances, not just those in a university clinic. Also important is the level that these parent-driven interventions produce improvements that make a difference clinically, not just in a statistical analysis.
Another important component of understanding what interventions will work for individuals with autism is knowledge about some of the behavioral characteristics that match well with different types of interventions. These are called moderators of outcome. In addition to IQ at start of intervention, now clinicians know that vocabulary and ability to intentionally communicate during toddler years can predict later outcome. 48 These moderators should now be incorporated into personalized decisions made about what type of interventions will lead to the best chance of improvements in different people, and will better inform treatment decisions. Teaching children with autism as a way to exchange knowledge may not always be effective, those children with autism without cognitive impairment still don’t always grasp the intentions of teaching.49 This calls for alternative methods of learning and teaching in those with autism.
This is a just a sampling of some of the 2015 research that has made an impact on the science of autism. Science can be incremental, it can be slow, but each year there is progress–studies that changes the way scientists think or how the community manages autism.

References
- Nordahl CW, Iosif AM, Young GS, et al. Sex differences in the corpus callosum in preschool-aged children with autism spectrum disorder. Molecular autism. 2015;6:26.
- Schaer M, Kochalka J, Padmanabhan A, Supekar K, Menon V. Sex differences in cortical volume and gyrification in autism. Molecular autism. 2015;6:42.
- Wolff JJ, Gerig G, Lewis JD, et al. Altered corpus callosum morphology associated with autism over the first 2 years of life. Brain : a journal of neurology. 2015;138(Pt 7):2046-2058.
- Hiller RM, Young RL, Weber N. Sex differences in pre-diagnosis concerns for children later diagnosed with autism spectrum disorder. Autism : the international journal of research and practice. 2015.
- Messinger DS, Young GS, Webb SJ, et al. Early sex differences are not autism-specific: A Baby Siblings Research Consortium (BSRC) study. Molecular autism. 2015;6:32.
- Harrop C, Gulsrud A, Kasari C. Does Gender Moderate Core Deficits in ASD? An Investigation into Restricted and Repetitive Behaviors in Girls and Boys with ASD. Journal of autism and developmental disorders. 2015;45(11):3644-3655.
- Hu VW, Sarachana T, Sherrard RM, Kocher KM. Investigation of sex differences in the expression of RORA and its transcriptional targets in the brain as a potential contributor to the sex bias in autism. Molecular autism. 2015;6:7.
- Turner TN, Sharma K, Oh EC, et al. Loss of delta-catenin function in severe autism. Nature. 2015;520(7545):51-56.
- Frazier TW, Youngstrom EA, Hardan AY, Georgiades S, Constantino JN, Eng C. Quantitative autism symptom patterns recapitulate differential mechanisms of genetic transmission in single and multiple incidence families. Molecular autism. 2015;6:58.
- Sanders SJ, He X, Willsey AJ, et al. Insights into Autism Spectrum Disorder Genomic Architecture and Biology from 71 Risk Loci. Neuron. 2015;87(6):1215-1233.
- Gockley J, Willsey AJ, Dong S, Dougherty JD, Constantino JN, Sanders SJ. The female protective effect in autism spectrum disorder is not mediated by a single genetic locus. Molecular autism. 2015;6:25.
- Ozonoff S, Young GS, Landa RJ, et al. Diagnostic stability in young children at risk for autism spectrum disorder: a baby siblings research consortium study. Journal of child psychology and psychiatry, and allied disciplines. 2015;56(9):988-998.
- Zwaigenbaum L, Bryson SE, Brian J, et al. Stability of diagnostic assessment for autism spectrum disorder between 18 and 36 months in a high-risk cohort. Autism research : official journal of the International Society for Autism Research. 2015.
- Zwaigenbaum L, Bauman ML, Fein D, et al. Early Screening of Autism Spectrum Disorder: Recommendations for Practice and Research. Pediatrics. 2015;136 Suppl 1:S41-59.
- Flanagan HE, Smith IM, Vaillancourt T, et al. Stability and Change in the Cognitive and Adaptive Behaviour Scores of Preschoolers with Autism Spectrum Disorder. Journal of autism and developmental disorders. 2015;45(9):2691-2703.
- Miller M, Iosif AM, Young GS, et al. School-age outcomes of infants at risk for autism spectrum disorder. Autism research : official journal of the International Society for Autism Research. 2015.
- Sanders SJ, Ercan-Sencicek AG, Hus V, et al. Multiple recurrent de novo CNVs, including duplications of the 7q11.23 Williams syndrome region, are strongly associated with autism. Neuron. 2011;70(5):863-885.
- De Rubeis S, He X, Goldberg AP, et al. Synaptic, transcriptional and chromatin genes disrupted in autism. Nature. 2014;515(7526):209-215.
- Gaugler T, Klei L, Sanders SJ, et al. Most genetic risk for autism resides with common variation. Nature genetics. 2014;46(8):881-885.
- Klei L, Sanders SJ, Murtha MT, et al. Common genetic variants, acting additively, are a major source of risk for autism. Molecular autism. 2012;3(1):9.
- Krumm N, Turner TN, Baker C, et al. Excess of rare, inherited truncating mutations in autism. Nature genetics. 2015;47(6):582-588.
- D’Gama Alissa M, Pochareddy S, Li M, et al. Targeted DNA Sequencing from Autism Spectrum Disorder Brains Implicates Multiple Genetic Mechanisms. Neuron.88(5):910-917.
- Simmons D. Epigenetic influence and disease. Nature Education. 2008;1(1).
- Roadmap Epigenomics C, Kundaje A, Meuleman W, et al. Integrative analysis of 111 reference human epigenomes. Nature. 2015;518(7539):317-330.
- O’Roak BJ, Vives L, Fu W, et al. Multiplex targeted sequencing identifies recurrently mutated genes in autism spectrum disorders. Science. 2012;338(6114):1619-1622.
- Neale BM, Kou Y, Liu L, et al. Patterns and rates of exonic de novo mutations in autism spectrum disorders. Nature. 2012;485(7397):242-245.
- Cotney J, Muhle RA, Sanders SJ, et al. The autism-associated chromatin modifier CHD8 regulates other autism risk genes during human neurodevelopment. Nature communications. 2015;6:6404.
- Feinberg JI, Bakulski KM, Jaffe AE, et al. Paternal sperm DNA methylation associated with early signs of autism risk in an autism-enriched cohort. International journal of epidemiology. 2015;44(4):1199-1210.
- Mazina V, Gerdts J, Trinh S, et al. Epigenetics of autism-related impairment: copy number variation and maternal infection. Journal of developmental and behavioral pediatrics : JDBP. 2015;36(2):61-67.
- Leblond CS, Nava C, Polge A, et al. Meta-analysis of SHANK Mutations in Autism Spectrum Disorders: a gradient of severity in cognitive impairments. PLoS genetics. 2014;10(9):e1004580.
- Lukose R, Beebe K, Kulesza RJ, Jr. Organization of the human superior olivary complex in 15q duplication syndromes and autism spectrum disorders. Neuroscience. 2015;286:216-230.
- Wegiel J, Flory M, Schanen NC, et al. Significant neuronal soma volume deficit in the limbic system in subjects with 15q11.2-q13 duplications. Acta neuropathologica communications. 2015;3(1):63.
- Gupta S, Ellis SE, Ashar FN, et al. Transcriptome analysis reveals dysregulation of innate immune response genes and neuronal activity-dependent genes in autism. Nature communications. 2014;5:5748.
- Chana G, Laskaris L, Pantelis C, et al. Decreased expression of mGluR5 within the dorsolateral prefrontal cortex in autism and increased microglial number in mGluR5 knockout mice: Pathophysiological and neurobehavioral implications. Brain, behavior, and immunity. 2015;49:197-205.
- van Bon BW, Coe BP, Bernier R, et al. Disruptive de novo mutations of DYRK1A lead to a syndromic form of autism and ID. Molecular psychiatry. 2015.
- Tropea D, Giacometti E, Wilson NR, et al. Partial reversal of Rett Syndrome-like symptoms in MeCP2 mutant mice. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(6):2029-2034.
- Sztainberg Y, Chen HM, Swann JW, et al. Reversal of phenotypes in MECP2 duplication mice using genetic rescue or antisense oligonucleotides. Nature. 2015.
- Yi JJ, Berrios J, Newbern JM, et al. An Autism-Linked Mutation Disables Phosphorylation Control of UBE3A. Cell. 2015;162(4):795-807.
- Estes A, Munson J, Rogers SJ, Greenson J, Winter J, Dawson G. Long-Term Outcomes of Early Intervention in 6-Year-Old Children With Autism Spectrum Disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2015;54(7):580-587.
- Yatawara CJ, Einfeld SL, Hickie IB, Davenport TA, Guastella AJ. The effect of oxytocin nasal spray on social interaction deficits observed in young children with autism: a randomized clinical crossover trial. Molecular psychiatry. 2015.
- Hyman SL, Stewart PA, Foley J, et al. The Gluten-Free/Casein-Free Diet: A Double-Blind Challenge Trial in Children with Autism. Journal of autism and developmental disorders. 2015.
- Harris H, Israeli D, Minshew N, et al. Perceptual learning in autism: over-specificity and possible remedies. Nature neuroscience. 2015;18(11):1574-1576.
- Johnson CP, Myers SM, American Academy of Pediatrics Council on Children With D. Identification and evaluation of children with autism spectrum disorders. Pediatrics. 2007;120(5):1183-1215.
- Zwaigenbaum L, Bauman ML, Choueiri R, et al. Early Identification and Interventions for Autism Spectrum Disorder: Executive Summary. Pediatrics. 2015;136 Suppl 1:S1-9.
- Green J, Charman T, Pickles A, et al. Parent-mediated intervention versus no intervention for infants at high risk of autism: a parallel, single-blind, randomised trial. The lancet. Psychiatry. 2015;2(2):133-140.
- Bearss K, Johnson C, Smith T, et al. Effect of parent training vs parent education on behavioral problems in children with autism spectrum disorder: a randomized clinical trial. Jama. 2015;313(15):1524-1533.
- Gulsrud AC, Hellemann G, Shire S, Kasari C. Isolating active ingredients in a parent-mediated social communication intervention for toddlers with autism spectrum disorder. Journal of child psychology and psychiatry, and allied disciplines. 2015.
- Yoder P, Watson LR, Lambert W. Value-added predictors of expressive and receptive language growth in initially nonverbal preschoolers with autism spectrum disorders. Journal of autism and developmental disorders. 2015;45(5):1254-1270.
- Knutsen J, Mandell DS, Frye D. Children with autism are impaired in the understanding of teaching. Developmental science. 2015.
- Baller JB, Barry CL, Shea K, Walker MM, Ouellette R, Mandell DS. Assessing early implementation of state autism insurance mandates. Autism : the international journal of research and practice. 2015.
