
Common coqui frog male (Eleutherodactylus coqui, snout-to vent length average ~ 3 cm) camouflaged in the fronds of an epiphyte in the El Yunque National Forest (Puerto Rico), along with an image of the enchanted forest of the Sierra de Luquillo where Narins & Meenderink did their study (4) – photos courtesy of Thomas Fletcher. This species can be found from sea level to the top of the highest peak in Puerto Rico (Cerro Punta = 1338 m). Native to mesic ecosystems, common coquis are well adapted to a terrestrial life, e.g., they lack interdigital webbing that support swimming propulsion in many amphibians, and youngsters hatch directly from the egg without transiting a tadpole stage. The IUCN catalogues the species as ‘Least Concern’ though alerts recent declines in high-altitude populations caused by chytrid fungus – lethal to amphibians at a planetary scale (9). Remarkably, the species has been introduced to Florida, Hawaii, the Dominican Republic and the Virgin Islands where it can become a pest due to high fertility rates (several >20 egg clutches/female/year).
Frog songs are species-specific and highly useful for the study of tropical communities, which host the highest amphibian diversities globally. The auditory system of females and the vocal system of males have co-evolved to facilitate reproductive encounters, but global warming might be disrupting the frequency of sound-based encounters in some species..
It is a rainy night, and Don (Gene Kelly) has just left his love, Kathy (Debbie Reynolds), at home, starting one of the most famous musical movie scenes ever: Singin’ in the rain …
Amphibians (see Amphibians for kids by National Geographic) also love to sing in rainy nights when males call for a partner, but now they have to do it in hotter conditions as local climates become warmer. Vocal behavior is a critical trait in the life history of many frog species because it mediates recognition between individuals, including sexual selection by females (1).
With few exceptions, every species has a different and unique call, so scientists can use call features to identify species, and this trait is particularly useful in the inventory of diverse tropical communities (2). Differences in call frequency, duration and pitch, and in note, number, and repetition pattern, occur from one species to another. And even within species, songs can vary from individual to individual (as much as there are not two people with the same voice), and be tuned according to body size and environmental temperature (3).
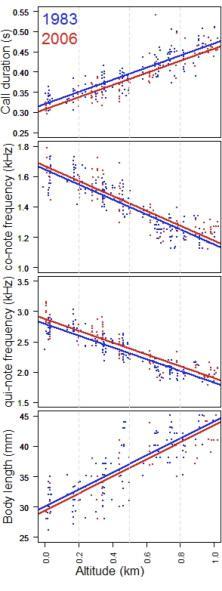
Male vocalisations of common coqui males along a 1-km altitudinal gradient in the Sierra de Luquillo (Puerto Rico) in 1983 and 2006 (4). The duration of individual vocalizations (in seconds = co & qui notes together) and the frequency of individual notes (in kHz = co or qui notes separately) were averaged over 3 to 23 vocalisations/male in a sample of > 100 individuals. All males were measured from snout to vent (in mm). In the graphs, each point represents a unique male and linear trends capture the relationship between altitude, body size and song features. Body size and call duration grow, and note frequency wanes, as altitude increases at Luquillo. Change rates in body size and song features are of the same magnitude in both study years (i.e., parallel annual trends). However, at any given altitude relative to 1983, 2006 males were smaller and emitted shorter songs (2006 below 1983 trend) with higher-frequency notes (2006 above 1983 trend).
Of course, as with most ectotherms, amphibians regulate their body temperature in response to environmental temperature, but both the emission and reception of vocalisations also depend on thermal context (4, 5). In fact, there can be a temperature-determined coupling between how females hear a male’s song and how that male vocalises — to the extreme that females can select for reproductive partners singing at a similar body temperature (6).
Using the former functional relationship, Peter Narins & Sebastiaan Meenderink monitored both in 1983 and 2006 the vocal behavior and body size of common coqui frogs Eleutherodactylus coqui along a gradient of altitude (10 to 1000 metres) in the Sierra de Luquillo, Yunque National Forest, Puerto Rico (7).
At dusk and early night, male coquis (see and listen here) fill the Luquillo temple of trees with their two-note call, which sounds literally like a co followed by a qui, the two notes being repeated over and over. Interestingly, the first note (co) plays a role in territorial defense against rival males, while the second note (qui) is used to attract potential female mates (8).
Narins & Meenderink found that for the altitudes at which they recorded a given male body size and a given song duration (co+qui) in 2006, those body sizes and call durations had been at an altitude 47 and 87 metres lower in 1986. In contrast, the frequency of both notes had climbed 49 and 83 metres over the same stretch of time, respectively.
During the study period, temperature records from four local meteorological stations revealed a thermal increase by 0.5 °C along the slopes of the Sierra de Luquillo — which matched the observed shift in frog vocalisations that would be expected by metabolic-physiological theory (9).
Most importantly, the ~ 34-metre difference in how co and qui notes have shifted in altitude between 1986 and 2006 is bound to decouple females from vocal males appropriate for reproduction (6). How common this phenomenon might be in frogs, and how strongly it could affect population fitness, are unknown. However, since temperature increases can stimulate vocal activity in some species and inhibit it in others (9), acoustic responses to warming are expected to be shaped by species-specific life histories and distribution along environmental gradients.
—
by David R. Vieites & Salvador Herrando-Pérez
(with support of the British Ecological Society and the Spanish Ministry of Economy, Industry and Competitiveness)
—
References
- Gerhardt, HC (1994) The evolution of vocalization in frogs and toads. Annual Review of Ecology and Systematics 25: 293-324
- Vieites, DR et al. (2009) Vast underestimation of Madagascar’s biodiversity evidenced by an integrative amphibian inventory. Proceedings of the National Academy of Sciences of the USA 106: 8267-8272
- Gayou, DC (1984) Effects of temperature on the mating call of Hyla versicolor. Copeia 3: 733-738
- Navas, CA (1996). The effect of temperature on the vocal activity of tropical anurans: a comparison of high and low-elevation species. Journal of Herpetology 30: 488-497
- Stiebler, IB & PM Narins (1990) Temperature-dependence of auditory nerve response properties in the frog. Hearing Research 46: 63-81
- Gerhardt, HC (1978) Temperature coupling in the vocal communication system of the gray tree frog, Hyla versicolor. Science, 199: 992-994
- Narins, PM & SW Meenderink (2014) Climate change and frog calls: long-term correlations along a tropical altitudinal gradient. Proceedings of the Royal Society of London B 281: 20140401
- Narins, PM & RR Capranica (1976) Sexual differences in the auditory system of the tree frog Eleutherodactylus coqui. Science 192: 378-380
- Ospina, OE et al. (2013) Variable response of anuran calling activity to daily precipitation and temperature: implications for climate change. Ecosphere 4: 1-12

