I just returned from my first San Antonio Breast Cancer Symposium which was an information-packed week of talks, posters, and meeting interesting people. I had a blast, learned a ton, and made a valuable connection at a company for a future study. The next few posts on my blog are going to cover the biggest news items as well as what I personally found the most interesting at the meeting. But outside of these topics, if you have any questions, feel free to comment and maybe I’ll add a post or just get back to you on it. The online resources available to the attendees of the meeting are vast so even if I didn’t attend that session, I should be able to get an answer for you from them or my twitter colleagues who attended.
I will kick off this short series of posts, with what I thought was the most promising new therapeutic advance, and this is the latest analysis of the trial of Pfizer’s CDK4/6 inhibitor, PD-0332991. When I was reading up on drugs for the book chapter I just wrote on cell cycle targeted therapies, apart from my own drug of interest, I was most excited about this compound. And indeed, the clinical results of a randomized phase 1/2 study presented by Richard Finn were stunning! The progression-free survival curve presented is shown in figure 1. “LET” stands for letrozole, a non-steroidal aromatase inhibitor, an FDA approved drug that is currently used in post-menopausal patients with hormone-sensitive tumors.
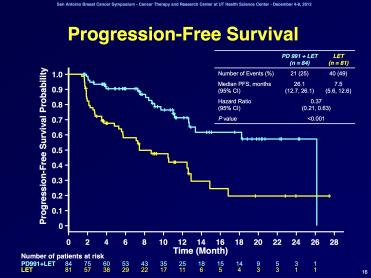
Figure 1: Progression-free survival curves for letrozole vs PD+letrozole in postmenopausal ER+ patients.
To elaborate on this study, which was a worldwide multi-center trial of postmenopausal ER+, HER2- patients based on the preclinical work that highlighted that targeting CDK4/6 only works in the context of cells with intact G1 checkpoint (ie wild-type Rb). In case you are not familiar with the cell cycle, figure 2 has a schematic of the cell cycle and proteins involved in each phase.
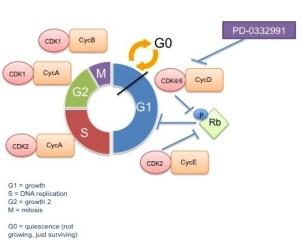
Figure 2: Cell cycle schematic
Note that CDK4 and CDK6, targets of this drug, function in complex with cyclin D1 to phosphorylate and inactivate Rb, which is a major cellular brake on cell proliferation. It would make sense then that targeting CDK4/6 in the context of tumors cells that have lost Rb would be ineffective. Since luminal (ER positive) breast cancers also frequently overexpress cyclin D1, Pfizer decided to focus on these patients, however they do have an additional 3 separate trials in lymphoma and myeloma, based on preclinical data in these models too.
This drug was incredibly well tolerated, with the majority of the AEs being grades 1 or 2. The only added toxicity over letrozole alone that was observed in more than a few patients was neutropenia, which was described by Dr Finn as “uncomplicated”, and expected for this class of compounds which do affect normal cells as well.
The results of the biomarker analysis was intruiging however for the basic scientists among us. Even though it was thought that cyclin D1 amplification or loss of p16 (which is a negative regulator of CDK4/6 activity) would be predictive biomarkers of sensitivity, and were used to enrich the phase 2 part of this trial for potential responders (after phase 1 found the maximum tolerated dose). However, upon biomarker analysis at the end of the study, they found that these proteins were no better than ER alone at predicting response. Clearly, further work is necessary to find a biomarker of response (and/or resistance), even though the clinical benefit rate of 70% seen in this study was quite impressive. In conclusion though, based on these data (both preclinical and this study), a registration study is planned to start in 2013.
References (journal articles):
Preclinical breast cancer papers:
“PD 0332991, a selective cyclin D kinase 4/6 inhibitor, preferentially inhibits proliferation of luminal estrogen receptor-positive human breast cancer cell lines in vitro.” – Breast Cancer Res Treatment 2009, pubmed link
“Therapeutic response to CDK4/6 inhibition in breast cancer defined by ex vivo analyses of human tumors.” – Cell cycle 2012, pubmed link
Preclinical other tumors:
“A novel orally active small molecule potently induces G1 arrest in primary myeloma cells and prevents tumor growth by specific inhibition of cyclin-dependent kinase 4/6.” – Cancer Res 2006, pubmed link
“Pharmacologic inhibition of CDK4/6: mechanistic evidence for selective activity or acquired resistance in acute myeloid leukemia.” – Blood 2007, pubmed link
“Pharmacologic inhibition of cyclin-dependent kinases 4 and 6 arrests the growth of glioblastoma multiforme intracranial xenografts.” – Cancer Res 2010, pubmed link
“Selective CDK4/6 inhibition with tumor responses by PD0332991 in patients with mantle cell lymphoma” – Blood 2012, pubmed link
