
Mounted models of the gamma helix and alpha helix, as housed in the Special Collections & Archives Research Center, Oregon State University Libraries.
[Part 2 of 3]
Linus Pauling sent shock waves through the scientific community when he published seven articles relating to the structure and function of proteins in the April-May 1951 issue of the Proceedings of the National Academy of Sciences. The first article of this volley was titled “The structure of proteins: Two Hydrogen-Bonded Helical Configurations of the Polypeptide Chain.” The second article was written by Pauling and Robert B. Corey, and was called “Atomic Coordinates and Structure Factors for Two Helical Configurations of Polypeptide Chains.” This paper was much more technical than was the first, and introduced two new important models developed by the Pauling group: the Gamma-helix and the Alpha-helix.
The article began by explaining in great detail Pauling’s idea for what he called the Gamma-helix (γ-helix). The γ-helix was the name assigned to the 5.1-residue helical configuration that Pauling, Corey, and Herman Branson had described in the first PNAS proteins article. The main difference between the γ-helix and the other configurations that they had proposed was that the bond angle between the C-N-C connection had been changed from 123˚ to 120˚. The authors explained that this small adjustment in the bond angle resulted in a miniscule change in the interatomic distances between various hydrogen bonds, but that these changes were significant enough to notably affect the number of residues per turn present within the structure.
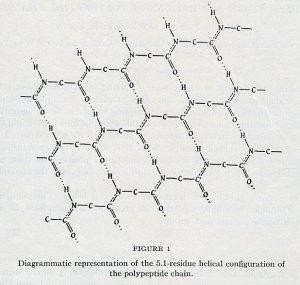
(It is worth noting that the unit used to measure the distance between molecules is the Angstrom (Å). One Å equals 10-10 m, or one ten-billionth of a meter. Considering the truly tiny sizes being measured, it is likewise worth noting that the changes between hydrogen bonds that Pauling was talking about were often measured in the thousandths of an Angstrom or ten-trillionths of a meter. One trillionth of a meter is known as a picometer.)
The article stressed that differences as small as 10 picometers could notably change bond angles, which would then change the number of residues per turn, thus dramatically affecting the shape of the helix. Next, the authors elaborated upon the likely arrangements of molecules within the helix due to symmetry or lack of symmetry in certain molecular bonds. Pauling and Corey further noted that they had used x-ray crystallography to validate their arguments and determine the crystal structures in question. From his earliest days as a scientist, Pauling had established himself as a major figure in x-ray crystallography, a technique by which an operator fires x-rays at a substance in question, then measures the way that the x-rays have deflected off of the substance. By analyzing these deflection patterns, researchers were then able to develop models of the shapes of molecular structures.
Once the γ-helix had been explained, the article moved on to discuss the Alpha-helix (α-helix). The group explained that the γ-helix and the α-helix were similar in terms of how the hydrogens bonded with other molecular groups, and how the residues fit under those configurations. They also detailed the exact interatomic distances between hydrogen and various other molecules in the structure, while pointing out that the distance between carbon and its other bonds determined the number of residues per turn. The number of turns, however, was variable; the smallest possible angle of 108.9˚ resulted in a residue of 3.6, while the largest possible angle of 110.8˚ resulted in a 3.67 residue.
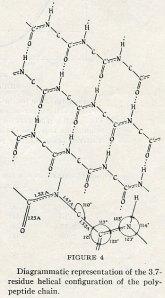
William Lawrence Bragg, an internationally famous scientist and proteins researcher of great import, was impressed by the α-helix, though he felt his rival Pauling to be overly excited about it. The α-helix was not a complete protein, except in a few cases including fibers, hair, and horn. The structure also did not explain the functioning of proteins. As such, Bragg felt the paper to be an important first step – no more, no less. The rest of the scientific community was more enthusiastic than was Bragg and his team. Pauling received a Nobel Prize in 1954 in Chemistry “for his research into the nature of the chemical bond and its application to the elucidation of the structure of complex substances,” the α-helix being among the most famous of these “complex structures.” The National Science Foundation even named a research vessel The Alpha Helix in honor of the discovery.
Bragg was even less generous regarding the γ-helix. Though in his very carefully worded congratulatory letter to Pauling he did not say so, he felt the γ-helix to be far-fetched, perhaps existing only in Pauling’s imagination. While this was not the case, the γ-helix ultimately made less of an impact on the scientific community.
Regardless, the import of Pauling’s work was felt throughout the profession. Though Francis Crick would write that the alpha helix did not give him and Jim Watson the idea that DNA was a double helix, he did suggest that “helices were in the air,” at the time “and you would have to be either obtuse or very obstinate not to think along helical lines.”
