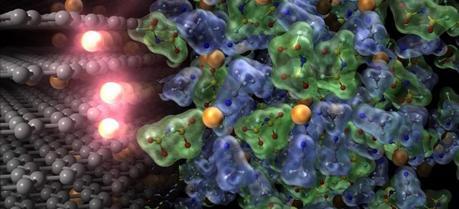
 Charging reaction of lithium-ion batteries. Lithium ions (orange spheres) move from the electrolyte (right) to the anode (left). Ethylene carbonate solvent was required as electrolyte for this reaction, but the current research has made it possible to use a great variety of superconcentrated electrolytes as solvent. The new electrolyte developed in this research remarkably increases the reaction kinetics and thus contributes to the development of fast-charging lithium-ion batteries that can be charged in just one-third of the time of conventional batteries. (Credit: Yuki Yamada / University of Tokyo)
Charging reaction of lithium-ion batteries. Lithium ions (orange spheres) move from the electrolyte (right) to the anode (left). Ethylene carbonate solvent was required as electrolyte for this reaction, but the current research has made it possible to use a great variety of superconcentrated electrolytes as solvent. The new electrolyte developed in this research remarkably increases the reaction kinetics and thus contributes to the development of fast-charging lithium-ion batteries that can be charged in just one-third of the time of conventional batteries. (Credit: Yuki Yamada / University of Tokyo)Scientists in Japan have developed an innovative electrolyte that may be the key to the next great breakthrough in advanced lithium-ion batteries.
The three primary functional components of a lithium-ion battery are the positive and negative electrodes and electrolyte. Generally, the negative electrode of a conventional lithium-ion cell is made from carbon. The positive electrode is a metal oxide, and the electrolyte is a lithium salt in an organic solvent. The electrolyte is typically a mixture of organic carbonates such as ethylene carbonate or diethyl carbonate containing complexes of lithium ions.
Now, assistant Professor Yuki Yamada and Professor Atsuo Yamada at the University of Tokyo, Graduate School of Engineering, Department of Chemical System Engineering, Dr. Keitaro Sodeyama at Kyoto University, and Dr. Yoshitaka Tateyama at the National Institute of Materials Science have developed a new electrolyte for fast-charging and high-voltage lithium-ion batteries.
The new electrolyte is an organic solution containing a superhigh concentration of lithium ions, over four times more concentrated than current electrolytes. The group has discovered unusual stability and fast reaction kinetics in the superconcentrated electrolyte, far exceeding currently used commercial electrolytes. The group have revealed that the origin of these peculiar properties is due to a particular solution structure by molecular dynamics simulations using the K supercomputer at RIKEN.
The new electrolyte will open up the way to development of advanced lithium-ion batteries that can be charged in a third of the time of conventional lithium-ion batteries and deliver voltages much greater than the current limit of 3.7 V and as high as 5 V, which will enable their use in applications requiring high energy densities such as electric vehicles and smart grids.
Earlier this month we reported that researchers at the USC Viterbi School of Engineering had improved the performance and capacity of lithium batteries by developing better-performing, cheaper anode and cathode materials.
Yamada, Y., Furukawa, K., Sodeyama, K., Kikuchi, K., Yaegashi, M., Tateyama, Y., & Yamada, A. (2014). Unusual Stability of Acetonitrile-Based Superconcentrated Electrolytes for Fast-Charging Lithium-Ion Batteries Journal of the American Chemical Society, 136 (13), 5039-5046 DOI: 10.1021/ja412807w
