 The strong activity of the new catalyst material (dark circle at center) in promoting the oxygen evolution reaction when submerged in water, as revealed by the bubbles of oxygen forming on its surface. (Credit: MIT)
The strong activity of the new catalyst material (dark circle at center) in promoting the oxygen evolution reaction when submerged in water, as revealed by the bubbles of oxygen forming on its surface. (Credit: MIT)Scientists at the MIT have discovered a new family of materials (“double perovskites”) that provides the best-ever performance in a reaction called oxygen evolution. According to an MIT article, this is a key requirement for energy storage and delivery systems such as advanced fuel cells and lithium-air batteries.
Oxygen evolution is the process of generating molecular oxygen through a chemical reaction. Mechanisms of oxygen evolution include the oxidation of water during oxygenic photosynthesis, electrolysis of water into oxygen and hydrogen, and electrocatalytic oxygen evolution from oxides and oxoacids.
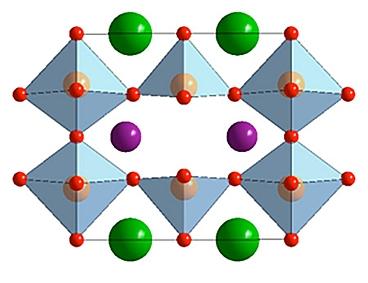
A diagram of the molecular structure of double perovskite shows how atoms of barium (green) and a lanthanide (purple) are arranged within a crystalline structure of cobalt (pink) and oxygen (red). (Credit: See citation at the end of this article)
Photosynthetic oxygen evolution is the fundamental process by which breathable oxygen is generated in the Earth’s biosphere. The reaction is part of the light-dependent reactions of photosynthesis in cyanobacteria and the chloroplasts of green algae and plants. It utilizes the energy of light to split a water molecule into its protons and electrons for photosynthesis. Free oxygen is generated as a by-product of this reaction, and is released into the atmosphere.
The materials, called double perovskites, are a variant of a mineral that exists in abundance in the Earth’s crust. Their remarkable ability to promote oxygen evolution in a water-splitting reaction is detailed in a paper appearing in the journal Nature Communications (see footnote). The work was conducted by Yang Shao-Horn, the Gail E. Kendall Professor of Mechanical Engineering and Materials Science and Engineering; postdoc Alexis Grimaud; and six others.
The specific compounds used in this research were made by combining a lanthanide (praseodymium, samarium, gadolinium or holmium) with barium, cobalt and oxygen. These compounds form a crystal structure with one distinct site for barium and another for the lanthanide.
The performance of the double perovskites, Shao-Horn says, is a step forward from the previous record-holder for a catalyst that promotes electrochemical water-splitting—a material that Shao-Horn and her team reported in a paper in Science two years ago. In addition, these double perovskites are stable unlike earlier pseudocubic perovskites with comparable activities which readily amorphized during the oxygen evolution reaction.
An inexpensive way to split water into its constituent elements is one of the keys to the much-discussed “hydrogen economy”—a proposed system of delivering energy using hydrogen. Hydrogen is seen as a potential fuel for cars, the energy needs of buildings and portable electronics. Since free hydrogen does not occur naturally in sufficient quantities, it must be generated by steam reformation of hydrocarbons, water electrolysis or other methods.
The MIT researchers predict that further research could lead to more-active catalysts for water-splitting. “We figured out what physical parameters could control the activity and stability” of the compounds, Grimaud says, providing guidance for future research. These compounds could find use in fuel cells, advanced rechargeable metal-air batteries, and direct-solar splitting of water, they say.
While some kinds of perovskite occur naturally, the compounds used in this study do not, Grimaud says. They are synthesized in the laboratory by a simple process: the scientists mix powders of the constituent materials, “grind them up and put it in a furnace at high temperature,” Grimaud says.
Grimaud A, May KJ, Carlton CE, Lee YL, Risch M, Hong WT, Zhou J, & Shao-Horn Y (2013). Double perovskites as a family of highly active catalysts for oxygen evolution in alkaline solution. Nature communications, 4 PMID: 24042731
