
The total score for this test is 10 marks. You should complete the test in 10 minutes.
Section A: Multiple Choice Questions (5 marks)
1. When hot hydrogen is passed over a black solid, the solid turned reddish brown. Which of the following could the black solid be?
A. copper (II) oxide
B. zinc oxide
C. magnesium oxide
D. calcium oxide
2. Underground steel pipes can be protected from rusting by sacrificial protection. Which element below is most suitable to be used as the sacrificial substance.
A. magnesium
B. carbon
C. lead
D. copper
3. An experiment is carried out to find the order of reactivity of some metals. Three metals, X, Y and Z are placed in separate solutions containing metal ions X, Y and Z.
The results from the experiment are shown below.
metal ion aqueous metal ion
X ion Y ion Z ion
X - No reaction observed. No reaction observed.
Y Reaction observed. - Reaction observed.
Z Reaction observed. No reaction observed. -
Arrange the metals according to their order of reactivity, starting from the most reactive.A. X, Y, Z
B. Y, Z, X
C. X, Z, Y
D. Y, X, Z
4. Which statement about metals is incorrect?
A. Metals can conduct electricity in the solid states.
B. Layers of atoms in pure metal can slide past one another easily.
C. Metallic bonding consist of positive ions in a sea of mobile electrons.
D. All metals react with water to form hydrogen.
5. Which of the reaction(s) will produce a gas?
reaction 1: sodium with water
reaction 2: heating calcium carbonate
reaction 3: copper with hydrochloric acid
A. 1, 2 and 3.
B. 1 and 2 only.
C. 1 and 3 only.
D. 2 only.
Section B: Open- ended questions (5 marks)
1. Which of the structure (X or Y) best represents
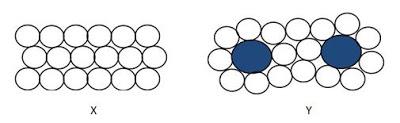
a. (i) solid pure metal
(ii) solid alloy
[2 marks]
b. Explain why is pure iron is seldom used industrially. [2]
c. Name an alloy of iron that does not rust. [1]
Answers
Section A:
1. A
2. A
3. B
4. D
5. B
Section B:
1 a. i) X (ii) Y
b. Pure metals are not strong enough. The regular arrangement of the atoms in pure metals, allow layers of atom to slide pass each other easily.
c. stainless steel
