Enantioselective Total Synthesis of (−)-Maoecrystal V
Regan J. Thompson et al., J. Am. Chem. Soc. 2014, 136, 17750 [PDF] [SI] [GROUP]
DOI: 10.1021/ja5109694


Maoecrystal V exhibits a heavily modified version of the more common ent-kaurene skeleton.
Interestingly, despite the hugely varied interests and specializations of the groups involved, all five of the successful total syntheses reported to date have constructed the molecule’s prominent bicyclo[2.2.2]octane ring system using the venerable Diels–Alder reaction (often in conjunction with the similarly tried-and-true tactic of oxidative dearomatization to establish the diene). That said, the number of Diels–Alder variants employed is impressive, and you could almost imagine giving a short lecture course on the reaction using nothing but examples from synthetic studies on maoecrystal V. I’ve tried to illustrate the variety below.
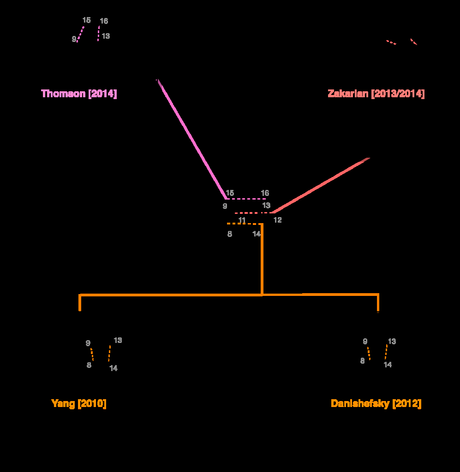
All 5 total syntheses to date have used a Diels–Alder reaction to form the molecule's fused bicyclo[2.2.2]octane ring system. The reaction has also featured prominently in approaches by Baran, Trauner, Nicolaou, Chen, Movin, Sorensen and others.[2]
I’ve long wanted to write something about maocrystal V total synthesis, but I’ve always been too busy around the time that people have completed it to get a blog post out reasonably close to the event. Fortunately, two back-to-back syntheses from the Zakarian and Thomson groups were published in J. Am. Chem. Soc. earlier this month and I’ve now got plenty time to write about both of them, starting with that of the Thomson group in this post.
The group began their studies with a commercially available cyclohexenone building block. A Baylis–Hillman reaction followed by a Sharpless epoxidation of the resulting allylic alcohol served to provide a source of asymmetry for the route. The alcohol was then coupled with a benzyl trichloroacetimidate, and the ketone was reduced to the secondary alcohol and thence converted to the iodide. Subsequent treatment of the iodide with zinc dust under acidic conditions resulted in fragmentation and rupture of the epoxide, and the resulting alcohol was temporarily masked with a TES protecting group. Next, a spectacular intramolecular Heck reaction linked the two rings, forming a new quaternary center with total stereocontrol.[3] Interestingly, the reaction gave a mixture of olefin products resulting from isomerization of the double bond, but fortunately the group largely obtained the desired product where the double bond had been transposed towards the gem-dimethyl group. In the same pot, both silyl protecting groups were removed in the reaction workup[4] and the team then proceeded to form their second ring in as many steps by an iodine(iii)-mediated oxidative dearomatization. Treatment of the phenol with di(acetoxy)iodobenzene resulted in trapping of the phenoxenium ion with the nearby cyclohexenol to close the central THF ring in near-quantitative yield. Finally, selective reduction of the less hindered dienone olefin with Stryker’s reagent proceeded in excellent yield.
Rapid generation of complexity through a Heck–oxidative dearomatization sequence
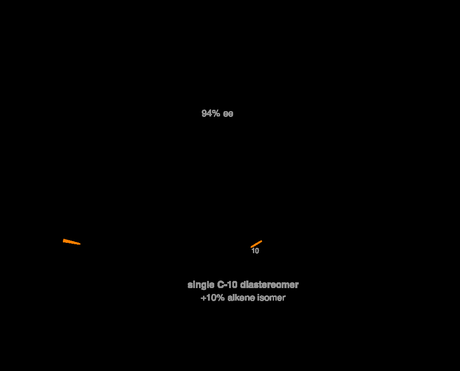
With their eyes now on the key Diels–Alder reaction the group converted the newly formed cyclohexenone to the corresponding diene by treatment with LDA and TMSCl. When this was treated with excess nitroethene a completely diastereoselective Diels–Alder reaction occurred, forming the expect ring system as the sole product in reasonable yield.[5],[6]

A completely diastereoselective Diels–Alder reaction completes the bicyclo[2.2.2]octane ring system. That's probably worth the hassle of preparing nitroethene.
With the molecule’s carbon skeleton now almost complete the group needed to convert their nitro group into a carbonyl and delete the unwanted ketone left over from their diene. First the ketone was converted to the dithiolane and the nitro group was carefully reduced to the corresponding amine with zinc/hydrochloric acid during the workup. This amine was then oxidized to the imine with IBX, and hydrolysis then gave the ketone. It's interesting to contrast this method of nitro-to-ketone interconversion with the horrendous set of conditions used by R.B.W. at the end of his erythromycin synthesis (NCS, Py; AgF, HMPA; H2O), although he had the added complication of needing selectivity for the oxidation of a secondary amine over three secondary alcohols.
Next, α-methylation of the newly formed ketone was required, but when this was performed though simple alkylation a 1:1 mixture of diastereomers at the new stereocenter was obtained. Fortunately, the group found a workaround where α-methylenation followed by reduction gave excellent selectivity (>15:1) for the required diastereomer. In practice, this involved another telescoped sequence wherein sodium borohydride was first used to carry out a conjugate reduction of the enone, and RaNi was then added to the reaction mixture to effect Mozingo-type desulfurization of the dithiolane. Finally, allylic oxidation of the cyclohexene to the cyclohexene proceeded in good yield under Wohl-Ziegler/Kornblum conditions.

Post-Diels–Alder adjustment of substituents on the bicyclo[2.2.2]octane and cyclohexene rings.
Now all that remained to complete the target was a C–H oxidation of one of the two tetrahydropyran methylene groups to form the lactone ring found in the natural product. Unfortunately, to cut a long story short, good selectivity for the required position could not be achieved, and the natural product was only obtained in 28% yield, along with a by-product resulting in oxidation at the unwanted position. It is interesting note how sensitive this reaction was to minor changes elsewhere in the molecule. For example, when the THP oxidation was attempted before oxidation of the cyclohexene to the cyclohexenone (i.e. if you swap the final and penultimate steps) then the undesired isomer was the sole product. Presumably, oxygenation of the lefthand ring serves to electronically deactivate the methylene nearest this position to the C–H activation, allowing some of the correct product to be formed. On the one hand, the surprising and dramatic sensitivity of these kinds of C–H oxidation steps to minor electronic effects does makes their use late on in a total synthesis a bit risky, but it also hints that we might one day be able to control them well enough to actually use them routinely—once we understand them a bit better.
Anyway, I think that congratulations are due to the Thomson group for an excellent synthesis and the successful execution of a bold last step.
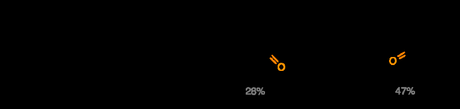
An audacious final C–H activation completes the target in a somewhat disappointing yield.
Addenda
- You might remember Sarah Reisman’s synthesis of Maocrystal Z was rightly named by Totally Synthetic as a contender for synthesis of the year back in 2011 and also lauded by Synthetic Nature. In fact, there are even more than that, because there are a number of different families of terpinoids produced and their biosynthetic progenitors, the kauranes and ent-kauranes, are also a large and storied family themselves. A number of reviews have summarizing the body of literature surrounding Maocrystal V. My favorite is the recent account in Angewandte by the Thomson group themselves (Angew. Chem. Int. Ed. 2014, 53, 10588).
- Interestingly, as far as I know only Dirk Trauner managed to build up this part of the molecule without recourse to the trusty D–A.
- PMP = 1,2,2,6,6-pentamethylpiperidine, or, if you’re eating it, pempidine.
- There are a lot of these telescoped steps in this route; a major factor to why it's so short. Although counting such operations as a single step for purposes of route length can be dishonest and misleading, and isn't something that I'm a fan of, I think that in this synthesis they're mostly justifiable as 1 pot operations. For example, in this instance the crude Heck reaction mixture is just diluted with THF, TBAF is added and the resulting mixture stirred for a while before work up.
- It's not too surprising that the diene chooses to approach from the opposite face to the tetrahydropyran ring. I'm a little bit more surprised that endo selectivity is so good, although the diastereoselectivity in this second regard is actually immaterial as the nitro group gets converted to a ketone later in the route.
- Unfortunately, nitroethene is not the sort of thing you can just buy from Aldrich, but I hear that it's not too bad to prepare—the usual method is simple dehydration of safe, commercial nitroethanol with phthalic anhydride. You often see it deployed as a reagent in benzene as it's best stored as a (frozen) benzene solution if you're not using it immediately: J. Org. Chem. 1980, 45, 1185.
