How biology is failing biology
I have spent a vast selection of my personal and professional time wondering about the one-dimensional representation of biological information and processes. I have also concluded that the representation of biological processes lacks very far behind other disciplines, such as engineering and computer science.
The notion is by no means novel, but after having read this article “Can a biologist fix a radio?—Or, what I learned while studying apoptosis“, it seemed as though the author had shared thoughts I had long harboured myself, albeit more articulately than I could likely have mustered.
The author of the above article uses the notion of fixing a broken radio to compare the diverging solutions that the disciplines of biology and engineering would likely employ. What fascinated me most, was the humour, but more importantly, clarity, with which he showcased the inefficiencies in the biologist’s evaluation of biological phenomena. I might add at this point, that being a biologist myself, I think it is okay for me to make this statement.
Biology is taught in one, or at most, two dimensions. Those of you who have been fortunate enough to pick up a biochemistry textbook in the last few decades, have been greeted by this, or similar, idyllic representation of a biological process:
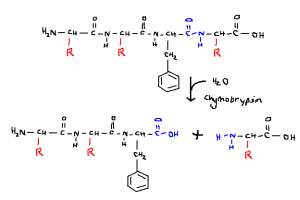
Cleavage at carboxyl end of hydrophobic amino acid, phenylalanine, by the enzyme chymotrypsin.
Though in principle, for teaching purposes, this is not wrong, nor is it wrong in any general sense. What I find particularly disconcerting is the implication of certainty that this action takes place at molecular level. Well it does. But not for all 100% percent of molecules present in any solution.
Agreeably, you might be thinking, enough with the ambiguity! Get to the point already!
Law of mass action and chemistry
Equilibrium chemistry is the study of the ratio at which products and reactants exist in a particular chemical solution, when that solution has reached equilibrium.
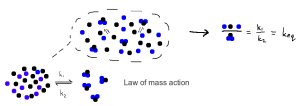
Law of mass action in equilibrium chemistry.
The law of mass action is the mathematical model concerned with understanding the likelihood that products would form, given an initial concentration of reactants. In the figure above, the ratio at which these components exist in solution after sufficient time has been allowed, is termed the equilibrium constant.
The effect therefore, of any compound, be it medicinal, nutritional, or hormonal, has a very strange road by which it affects the physiological equilibrium that is central to all of life. It is in fact the capacity to regulate this equilibrium, with enzymes and the separation of reactants from one another by subcellular compartmentation, that allows life to exist at all.
The biochemical reactions similar to the first figure is what you are used to seeing, and it is as one dimensional as saying that the presence of glucose in ones blood, increased the concentration of insulin, and therefore increases the absorption of glucose into the muscle/adipose tissue. Strictly speaking of course, this is not wrong, as this is just the 2nd grader summary of what really occurs.
In reality of course, the process still abides by the law of mass action and more fundamentally, the second law of thermodynamics. The nature of equilibrium chemistry above makes the reality of the simple blood glucose statement above, seem a very complicated process indeed. Indeed it is the proverbial equivalent of herding water.
The process above is more closely simulated by the diagram below.
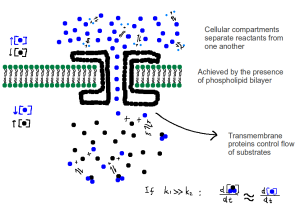
The control of reactants allows the exploiting of the second law of thermodynamics to sustain differential cellular equilibrium.
The second law of thermodynamics
The second law of thermodynamics states that all isolated systems are evolving towards a state of equilibrium (state of maximum entropy, or disorganized energy). It also states that the entropy of an isolated systems will spontaneously evolve towards a stable thermodynamic equilibrium.
Essentially, this means that if a cell, or subcellular compartment is irreversibly separated from its external environment, it will cease to live. It will reach thermodynamic equilibrium, and the only energy fluxes that occur will be the loss of heat energy. It is the continuous, and gradual shift in chemical equilibrium from extraneous sources that allows for the rate of energy flux to be larger than 0 (dE/dt > 0).
The impact
Ever wondered why there seem to be and infinite number diverging apparent solutions to common ailments? Antioxidants for the prevention of cardiovascular disease, low fat diet to reduce the risk of cardiovascular disease, increase exercise to reduce risk of cardiovascular disease. It is not a topic of discussion whether E=mc², is most appropriate law to use when the goal is building a nuclear reactor. Yet, in order to lose weight for example, there must be almost as many diets than there are individuals who are, at this very moment, trying to loose weight.
Why? It is because biology is poorly understood. The workings of biology is represented with visual flow diagrams, but because biology is dynamic in nature, the effects of the changes induced by the actions that modelled are not taken into account. The predictions that serve as foundations of biology, and therefore nutritional sciences, pharmaceutical sciences, and modern medicine, are based on assumed mechanisms and are therefore fundamentally flawed.
A solution to this problem?
The language of biology is not equipped to deal with this issue, but mathematics is. Calculus presents us with the ideas, philosophy and tools with which to analyse changes in equilibrium chemistry, with which to ultimately understand the magnitude of the impact that any one component can have on human physiology.
Should the flow diagrams in biology be replaced by system of differential equations, perhaps we could envision a biological research platform, where different results can be integrated with one another and mechanisms are not assumed for the sake of supporting correlation, and the inference of causality not committing the cardinal sin of statistically unwarranted extrapolation.


