 Send to Kindle&’
Send to Kindle&’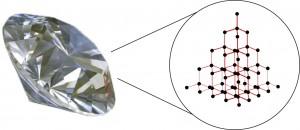 When people think of the C in the ABC’s of buying a diamond they automatically think of the 4 C’s, but we cover that plenty on this site and truth be told, we will cover that a ton more as time goes on, because that is truly the cornerstone of buying a diamond. But, what you don’t really learn about is the carbon, cell and crystal structure of a diamond.
When people think of the C in the ABC’s of buying a diamond they automatically think of the 4 C’s, but we cover that plenty on this site and truth be told, we will cover that a ton more as time goes on, because that is truly the cornerstone of buying a diamond. But, what you don’t really learn about is the carbon, cell and crystal structure of a diamond.
I have professed before and need to again, that I am a gem geek, and so this stuff fascinates me, and whenever I can add a bit of learning and history to the site, I’m going to slip it in! Buyer beware, learning is coming your way!!
C is for Carbon Atoms
The internal structure of the diamond is just like all other matter and is made up of tiny atoms, but they are arranged differently, which is what makes it into a mineral. Atoms have electrons, neutrons and protons. The protons and the neutrons form the center of the atom and the electrons orbit the protons and neutrons and surround it to form a shell. That is the basis for an atom, and the number of electrons in the outer shell of the atom is what determines the chemical nature of the atom and how it combines with other atoms.
You with me so far?
Carbon atoms have four outer electrons and they form covalent bonds, which are the strongest bonds that atoms can form. A covalent bond is a chemical bond formed by two atoms sharing electrons. These carbon atoms are connected in groups of five, with one in the center and the other four surrounding it. Each outside atom has a group of five surrounding it as well and so on and so on and these groups are called a tetrahedron.
C is for Unit Cell
The internal structure of a diamond is very uniform because they are built from what is called unit cells, which are the smallest group of atoms with the characteristic chemical composition and the basic crystal structure of a mineral. The core of the diamond’s unit cell is formed with four tetrahedrons, held together by shared electrons.
Unit cells continue to connect to build the crystal shapes or forms and the diamonds unit cell is cube-shaped, so it can actually be built to be many different shapes. Like building blocks, they can be stacked in many different ways.
C is for Crystal Structure
Regular repeating patterns of atoms form an internal arrangement called the crystal structure or crystal lattice and this is what affects the mineral’s characteristics. Diamonds are part of the cubic system and are the most symmetrical. They are the most well formed crystals and they are evenly proportioned and balanced.
Crystal structure is important because it affects the way that a diamond can be cut. In some directions, the crystal is harder and in some directions it is softer, so it is important for the cutter to understand the structure. The crystal structure also affects the density or weight per unit of volume, meaning they are heavier. Also crystal structure affects the behavior of light and the way that light will travel through the diamond based on the symmetry of the stone.
So, you can see how important it is to have a bit of background on each of these three C’s of diamonds: carbon atoms, unit cells and crystal structure, and now…
C is for Class Dismissed!
