Full post on this synthesis to come when I get back from holiday at the weekend and can use ChemDraw! Sorry for the hand-drawn stuctures - I've still not found a good way to draw stuctures on my iPad, which is all I have with me.
I've never been particularly excited by phloroglucinol-derived natural products, but I really enjoyed Shair's recent synthesis of the polyprenylation acylphloroglucinol (+)-hyperforin (it's a PPAP!), which appeared in the JACS ASAPs a few days ago.
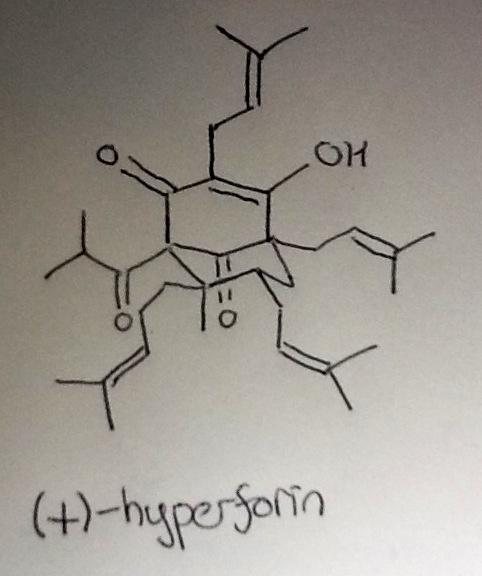
I'll hopefully get round to a more detailed discussion of the route in the next week, but the first step immediately caught my attention. The chemistry's simple enough - a good old-fashioned deprotonation step with t-BuLi, followed by treatment with barium(ii) iodide and prenyl chloride. I remembered (after looking it up) that the prenylation is done via the organobarium to improve the regioselectivity of the reaction, a trick developed a couple of decades back by the Yamamoto group (J. Am. Chem. Soc., 1994, 116, 6130).
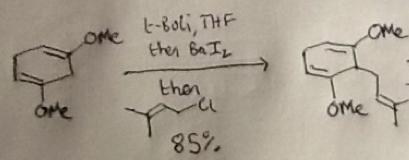
Still, I was curious about how the group carried out the reaction, and on what scale, so I delved into the SI (which, by the way, is excellent). There I found a slightly unusual tip for how to get the small barium pieces necessary to freshly prepare the BaI2:
"Using a hand drill hammer, a chisel, and a lead brick positioned on the laboratory floor, mineral oil-coated barium rod was portioned into approximately 25 mm segments... If a hand drill hammer and chisel are unavailable, a standard claw hammer and an appropriately-shaped shelving bracket may be employed in this step."
I'm not sure why, but seeing that in a JACS paper made me smile.
