
Image credit: Kanwal Jahan.
Colors convey ideas and emotions in such fundamental ways that being able to capture and use them has been an important component of both cultural and scientific development. The colors of the natural world have fascinated people throughout human history and unending attempts have been made to manipulate and apply color to the items that we use on a daily basis.
Linus Pauling was not immune to humankind’s curiosity for color and as a chemist he was intrigued by dye molecules. Seventy five years ago, in 1939, he attempted to deepen the scientific understanding of how these molecules reflect color.
By the late 1930s, chemists had become comfortable with the concept of electronic resonance – the ability of electrons in a molecule to change orbitals – and were using it to describe a molecule’s capacity to absorb and emit radiation in the reflection of color. Atoms and molecules possess electromagnetic radiation due to the charge of their electrons, and as light hits an atom or a molecule its radiation determines which wavelengths of light are absorbed and which are emitted. When a molecule resonates, the movement of electrons causes a shift in the charges within the molecule which affects its radiation and the distribution of its atoms. All of these processes impact the molecule’s absorption-emission spectra.
By the time that Linus Pauling began working with dyes he had already contributed greatly to the theory of resonance. In 1928, while looking at a series of proposed forms for resonating molecules, he realized that the likelihood that these molecules would resonate directly from one form to another was very low. While many of the resonance forms that had been proposed explained the chemical behavior of molecules, Pauling felt that something was missing in the contemporary understanding of resonance. In his 1928 paper, “The shared-electron chemical bond,” he proposed that the shifts in charge observed in larger molecules required intermediate resonance forms. Pauling then described how these shifts in charge occured from one atom to the next, in the process altering the molecule’s geometry. This idea ran contrary to the notion that electrons shifted directly from one side of the molecule to its opposite.
In 1939 Pauling applied these ideas to the molecules that make up dyes. Dye molecules are often large organic compounds highly affected by resonance. This fact was known to chemists at the time, yet Pauling disagreed with accepted ideas on how these compounds resonate and reflect color. To Pauling, it seemed unlikely that molecules the size and structure of, for example, benzaurin and indigo would resonate in such direct ways as was being proposed by his colleagues.
Although the dramatic changes in charge and structure that had been proposed did account for the colors reflected by dye molecules, Pauling had developed a different understanding of how they came about. Instead of electrons resonating and causing a shift in charge directly from one side of the molecule to the other, Pauling suggested that the shift occurred from atom to atom, giving rise to intermediate forms. Pauling believed that it was necessary to take into account all possible resonance forms in order to fully understand a dye’s emission spectrum.
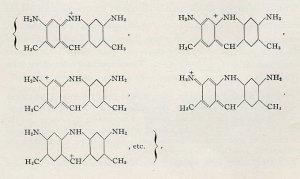
Some of the multiple resonance forms proposed by Pauling for Döbner’s violet. 1939.
Pauling’s thinking was published in a 1939 article, “A theory of the color of dyes,” which appeared in the Proceedings of the National Academy of Sciences. The article verifies the notion that color depends on the frequencies of radiation generated by the electrons in a molecule. But it also suggests that in order to understand their molecular structure and explain the colors that these molecules reflect, it is necessary to consider all possible distributions of a molecule’s charges, a combination of which would more accurately describe the observed reflection of color. Scientists now agree that understanding absorption-emission spectra is key in describing molecules because they offer valuable information about a molecule’s components and charges; Pauling’s dye work was a contribution to the development of this understanding.
At the time that Pauling’s theory of dyes paper was published, there were chemists across the country simultaneously trying to understand the color phenomenon. Dr. A. Burawoy’s 1940 article “Light Absorption, Resonance, and Isomerism” (Journal of the Society of Chemical Industry) used Pauling’s 1928 shared electron bond paper in developing his own study of dyes. Not surprisingly, Pauling and Burawoy reached similar conclusions about color.

Crellin Pauling and a friend peer out from a railroad car in an early color image from the Pauling Papers. Image digitized from a Kodachrome slide original.
Other chemists, including L.G.S. Brooker, would contribute to Pauling’s theory of dyes by questioning and expanding upon his work. Brooker was a chemist working for the Eastman Kodak Company in Rochester, New York. The company was naturally interested in producing higher-quality photographic film and, as such, was keen to investigate and understand the chemistry of dyes. Brooker and Pauling exchanged ideas as they studied dyes, and correspondence from December 1937 suggests that the two met in Rochester the following month to discuss their results. When Pauling’s theory of color was published in September 1939, Brooker wrote to issue a disagreement with Pauling’s treatment of carbon molecules. Specifically, Brooker believed that Pauling was overlooking the possible effects of carbon on a molecule’s behavior, though he otherwise agreed with Pauling’s conclusions on radiation and charge migration.
Observations like Brooker’s encouraged Pauling to continue his study of dyes by testing his theory on different molecules, including synthetic dyes like cyanine, which he investigated in 1940. The application of Pauling’s findings on carotenoids, one of the pigments found in tomatoes, was further expanded in a 1941 article published by Laszlo Zechmeister, Pauling and two other Caltech colleagues and titled, “Prolycopene, a naturally occurring stereoisomer of lycopene.” (Proceedings of the National Academy of Science) Two years later, Zechmeister, Pauling and three others authored “Spectral characteristics and configuration of some stereoisomeric carotenoids including prolycopene and pro-gamma-carotene.” (Journal of the American Chemical Society) Both publications explored the role of molecular structure in determining the emission spectra of naturally occurring pigments.
The contemporary understanding of how dye molecules reflect color has changed little since Pauling’s 1939 findings. His work, and that of many others scientists, confirms that something as simple as the color of a tomato is the result of a continuing cycle of complex interactions between atoms and their electrons.
