In the four months I spent not writing this post, the Paterson–Dalby synthesis of jiadifenolide was covered over at Synthetic Nature, but as I’d already put a few hours into it I decided to use the Christmas holidays to dust it off and finish it up. Enjoy! —BRSM
Total Synthesis of Jiadifenolide
I. Paterson et al., Angew. Chem. Int. Ed. 2014, 53, 7286–7289 [PDF] [SI] [Group]
The second synthesis in this two part series on jiadifenolide comes from the lab of Ian Paterson at Cambridge University in the UK, although it seems that Steven Dalby (now at Merck, Rahway) had enough of an impact on the work to also be named as a corresponding author. Like Sorensen’s approach, the British team also chose an “A-ring first” approach to the target, but instead of dipping into the chiral pool they instead built it up from simple 3-methyl-2-cyclopentenone through some clever use of a couple of highly diastereoselective rearrangements.
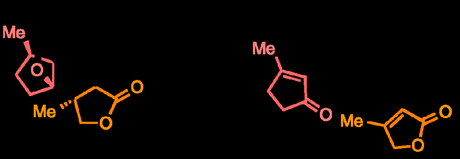
No fancy starting materials here!
The route began with a Luche reduction of the cyclopentenone,[1] followed up with a Henbest-style hydroxyl-directed epoxidation of the olefin with m-CBPA to form only the syn epoxy alcohol. Protection of the alcohol, followed by a Lewis-acid mediated 1,2-hydride shift formed the ketone with excellent diastereoselectively.[2] A highly E-selective HWE olefination then gave the enoate that was then reduced down to the allylic alcohol with lithium aluminum hydride and protected as the acetate. A Claisen–Ireland rearrangement then established the first of the molecule’s two all-carbon quaternary centers, again with good diastereoselectivity. Finally, removal of the TBS group under acidic conditions, followed by a Swern oxidation of the primary alcohol gave the ketoaldehyde.

The group functionalizes the natural product's A-ring with a number of highly diastereoselective transformations.
Now it’s probably fair to say that anyone with even the most superficial familiarity with Paterson’s oeuvre will no doubt be expecting an aldol reaction to appear in this route sooner or later, and that’s indeed what was used to couple their ketoaldehyde with the butenolide precursor to the C-ring.[3] After screening a large number of conditions, the group eventually identified a boron-mediated reaction that gave the coupled product in almost quantitative yield, albeit with only marginal 2:1 diastereoselectivity—and favoring the wrong epimer at C-11! However, crucially, the mixture could be easily separated chromatographically (after TES protection), so the two isomers could be studied separately in the key samarium(ii) iodide cyclization.
Unfortunately, to their frustration, the isomer bearing the correct hydroxyl configuration at C-11 found in the natural product did not undergo the planned cyclization at all, and attempts to use the oxidized C-11-keto derivative were similar unsuccessful. Luckily, it was eventually found that the epimeric C-11 TES ether with the wrong stereochemistry for the natural product—which was conveniently the major product of the previous aldol reaction anyway—did undergo the planned key step, giving the tricyclic product in moderate yield (but as a single diastereomer). Direct oxidation of the TES-protected C-11 alcohol to the ketone was then carried out using PCC, setting the stage for a diastereoselective reduction later on to hopefully obtain the required configuration at C-11. Interestingly, a small amount of over-oxidized product was also obtained from this reaction where, in addition to oxidation of the protected alcohol, an unexpected diastereoselective hydroxylation had occurred at the β-ketoester methine. This initially appeared to be a fortuitous discovery, as this unplanned hydroxylation was actually required to complete the natural product, and performing both oxidations in a single operation offered the tantalizing prospect of removing at least one step from the synthesis. Unfortunately, after further investigation the group were unable to obtain the doubly oxidized product in a synthetically useful yield, and so were forced to apply a separate hydroxylation to the mono-oxidized material.
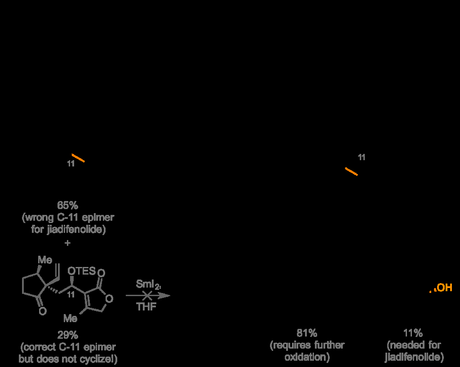
Samarium(ii) iodide effects an usual, diastereoselective cyclization to form the B-ring—and the molecule's second all-carbon quaternary center.
With the target’s complete carbon skeleton now laid down, the group began a final series of adjustments to the oxygenation pattern around the ring system. They began by taking the mono-oxidized β-ketoester left over from their previous reaction, and performed a Rubottom oxidation to obtain more of the diol that they needed to complete the synthesis. The newly installed hydroxyl was then used to direct a hightly diastereoselective reduction of the adjacent C-11 ketone using Evans’s tetramethylammonium triacetoxyborohydride reagent, finally setting this center with the correct stereochemistry. The resulting hydroxyl was temporarily masked with a TES group and dihydroxylation of the alkene then gave a diol that underwent an efficient oxidative lactonisation upon treatment with TPAP and NMO. Finally, removal of the TES group under acidic conditions resulted in spontaneous hemiacetal formation, completing the natural product.

A number of oxidation-level adjustments around the ring are then required to complete the target.
Although this is a diastereoselective synthesis, and it actually turned out several steps longer than Sorensen’s enantioselective route (largely due to the difficulties surrounding the key samarium(ii) iodide-mediated step), there’s plenty of interesting chemistry here. Also, at 2.3% overall, it’s comfortably the highest yielding route to jiadifenolide published so far, although I wouldn’t be surprised if we see one or two more in future. Well done to the team on a nice piece of work!
Addenda
- Although not ludicrously expensive (~ $10/g at time of writing) the group apparently prepared their starting material in-house from the much cheaper hexane-2,5-dione—not 2,5-pentanedione as they state in their paper. If you ever need one, there’s a beautifully detailed 5-page paper on exactly this one step synthesis by the Tsanaktsidis group (the people who also brought you the world’s most scalable cubane synthesis).
- I guess the product, tactic and conditions are reminiscent of Michael Jung’s famous-in-certain-circles non-aldol aldol reaction.
- No crazy 1,6 substrate-controlled stereoinduction here, though.
